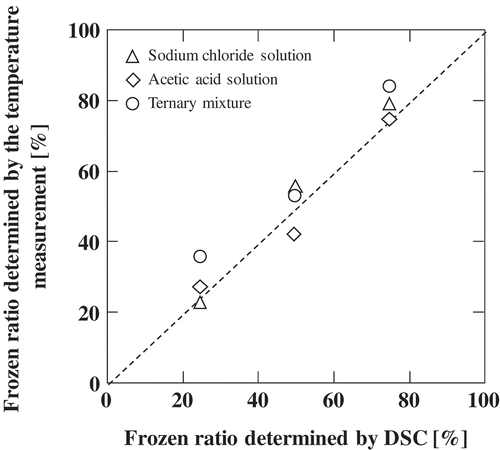
Figures & data
Figure 1. The DSC curves for various solutions. The weight ratios of sodium chloride to water are (A) 3:10, (B) 2:10, (C) 1:10, (D) 0.5:10, (E) 0.1:10, and (F) 0:10. The weight ratios of acetic acid to the sodium chloride aqueous solutions are (a) 5:5, (b) 4.5:5.5, (c) 4:6, (d) 3.5:6.5, (e) 3:7, (f) 2.5:7.5, (g) 2:8, (h) 1.5:8.5, (i) 1:9, (j) 0.5:9.5, and (k) 0:10.
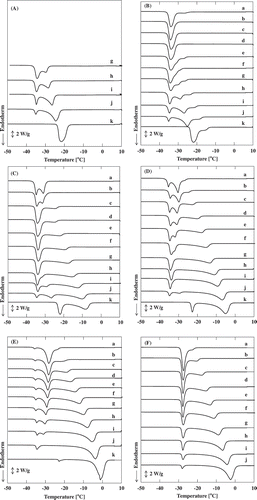
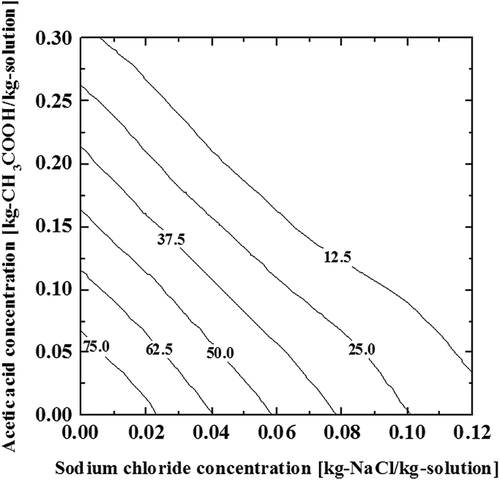
Figure 2. Liquid–solid phase equilibrium of the ternary aqueous solution of acetic acid and sodium chloride (a) equilibrium freezing point, (b) binary eutectic, and (c) ternary eutectic. The numbers on the contour lines indicate the temperature.
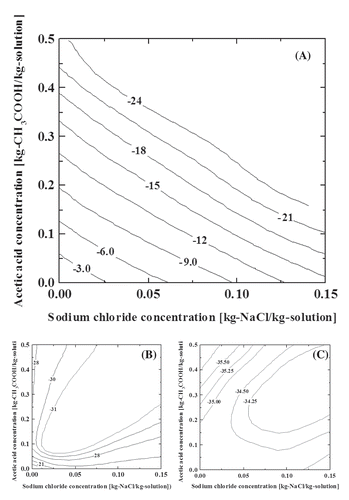
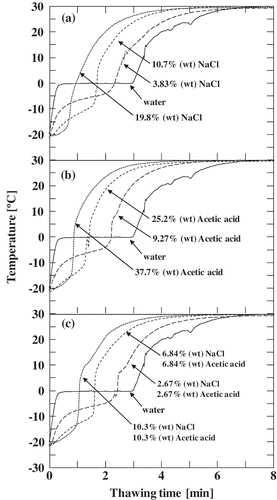
Figure 3. Temperature measurement during the thawing of (a) sodium chloride solution, (b) acetic acid solution, and (c) ternary mixture.