Figures & data
Table 1. Purification steps for phytase from Weissella halotolerans.
Figure 1. (a) Elution profile of Q-Sepharose anion exchange chromatography. The Q-Sepharose anion exchange column was equilibrated with 50 mM pH 5.5 sodium acetate buffer. Gradient elution was carried out by using 0–1 M NaCI. (b) Elution profile of Sephadex G-100 gel filtration column chromatography. The Sephadex G-100 gel was equilibrated with 50 mM pH 5.5 sodium acetate buffer. The elutions obtained the Q-Sepharose column was applied to the Sephadex G-100 column for purification.
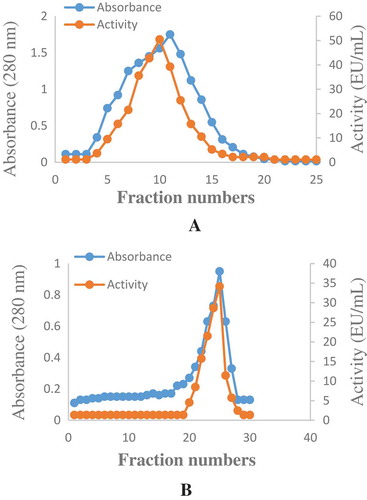
Figure 2. Optimum and pH stability studies were carried out by using 100 mM sodium acetate (pH 2.0–6.0), Tris–HCI (pH 7.0–9.0) and sodium carbonate (pH 10.0–12.0) buffer solutions. a-) Optimum pH profile for the purified phytase b-) pH Stability study for the purified phytase.
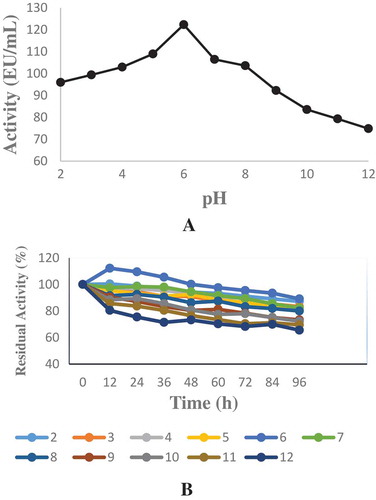
Figure 3. The optimum and temperature stability were determined between at 10°C and 90°C and sodium phytate was used as a substrate. a-) Optimum temperature profile for the purified phytase b) Temperature stability study for the purified phytase.
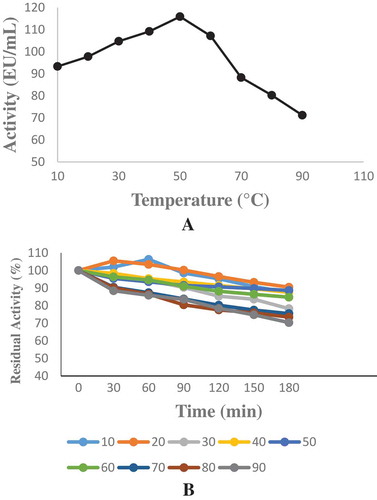
Figure 4. 1st, 2nd and 3rd bands are phytase enzyme on SDS-PAGE. The bands are the ammonium sulphate precipitation of the phytase enzyme solution and standard proteins in 5th and 6th well, respectively. The electrophoresis method was carried out according to the Laemmli’s procedure.
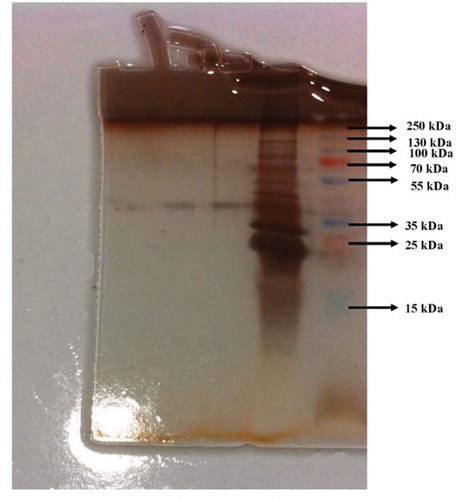
Table 2. The effects of metal ions on phytase purified from Weissella halotolerans.
