Figures & data
Table 1. Fatty acid composition of palm stearin (PS) and its fractions.
Table 2. Triacylglycerol composition of palm stearin and its fractions.
Table 3. Iodine value (IV) and slip melting point (SMP) of PS and its fractions.
Figure 1. Solid fat content vs. temperature profile of palm stearin (PS) and all fractions following fractionation at 30°C, 35°C, or 40°C (n = 3).
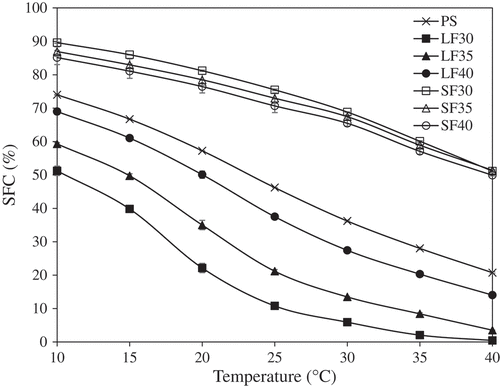
Table 4. Kinetic parameters associated with the isothermal crystallization of PS and all fractions using the Gompertz equation.
Figure 2. Putative crystallization curves for PS and all fractions during isothermal crystallization at (a) 24°C, (b) 29°C, and (c) at 40°C.
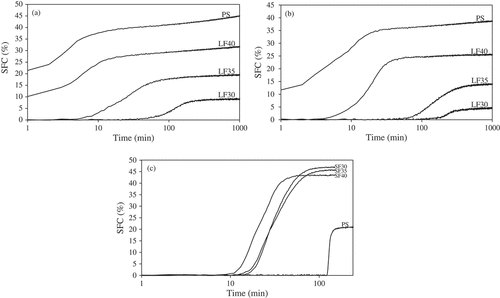
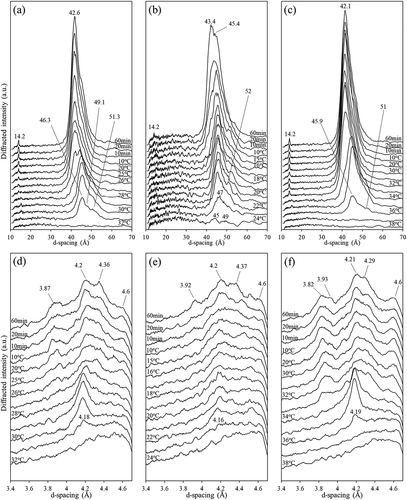
Figure 3. Crystal morphology of PS and all fractions (LF: liquid fraction; SF: solid fraction) obtained after quiescent crystallization at 24°C (top row) and 29°C (middle row) for 20 h or at 40°C (bottom row) for 4 h. PS: (a), (e), (i); LF30: (b), (f); LF35: (c), (g); LF40: (d), (h); SF30 (j); SF35 (k), and SF40 (l).
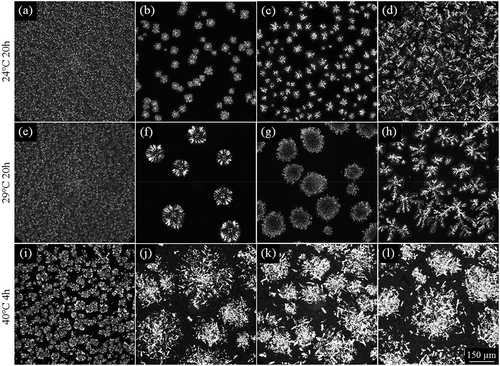
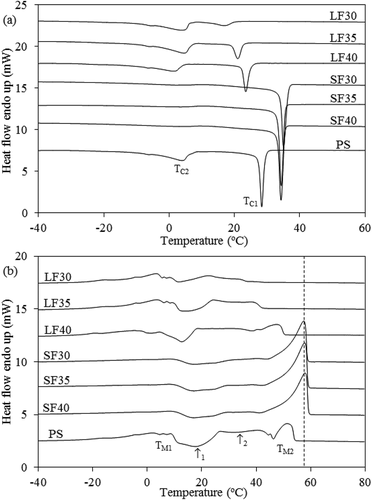
Table 5. Crystallization and melting transition temperatures and enthalpies for PS and all fractions.