Figures & data
Figure 1. Typical AFM height images of SA self-assembly in six different concentrations at 512 × 512 pixels. Scale bar: 500 nm. Concentration of SA solutions: (a) 0.001 mg/ml; (b) 0.005 mg/ml; (c) 0.02 mg/ml; (d) 0.05 mg/ml; (e) 0.08 mg/ml; (f) 0.10 mg/ml.
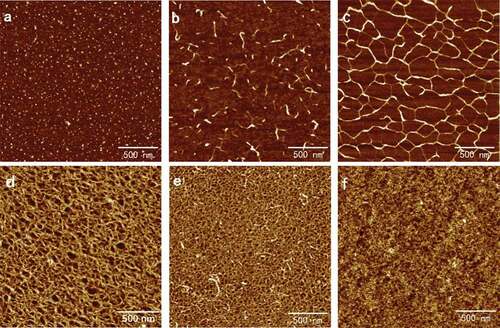
Figure 2. Root mean square roughness (Rq) of the self-assembly images of SA collected in different concentration.
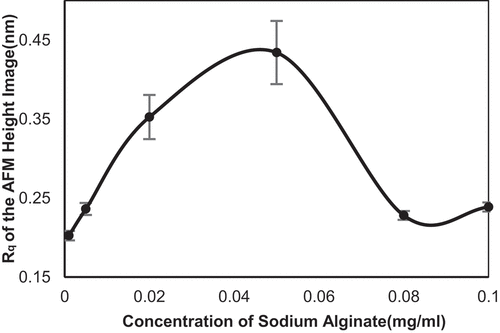
Figure 3. Typical AFM height images of 0.02 mg/ml SA self-assembly in CaCl2 solutions with different ions concentration in 512 × 512 pixels. Scale bar: 500 nm. Concentration of CaCl2 solutions: (a) 0.1 mmol/l; (b) 1 mmol/l; (c) 2 mmol/l; (d) 3 mmol/l; (e) 5 mmol/l; and (f) 10 mmol/l.
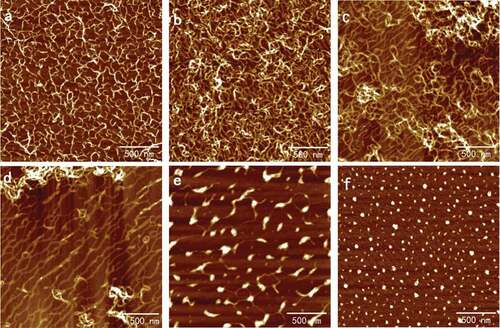
Figure 4. Typical AFM height images of 0.10 mg/ml SA self-assembly in presence of CaCl2 solutions with different concentration in 512 × 512 pixels. Scale bar: 500 nm. Concentration of CaCl2 solutions: (a) 0.1 mmol/l; (b) 1 mmol/l; (c) 2 mmol/l; (d) 3 mmol/l; (e) 5 mmol/l; and (f) 10 mmol/l.
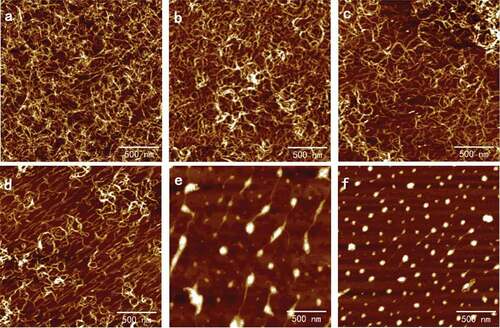
Figure 5. The diameter and density of the network holes and particles of SA self-assembly interacted with calcium chloride in different concentrations. (a) The diameter of the holes; (b) the density of the holes; (c) the diameter of the particles; and (d) the density of the particles.
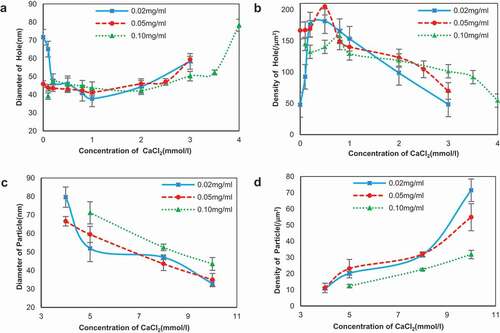
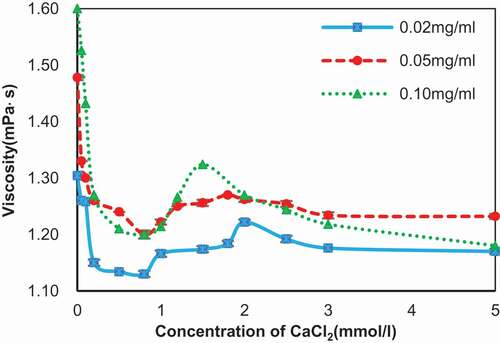
Figure 6. Root mean square roughness (Rq) of the self-assembly images of SA changed along with different concentration of calcium chloride. Concentration of SA: 0.02 mg/ml (blue line), 0.05 mg/ml (red line), and 0.10 mg/ml (green line).
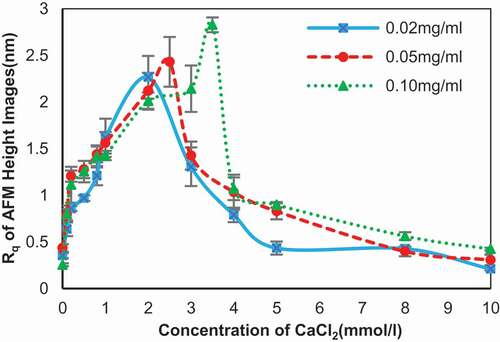
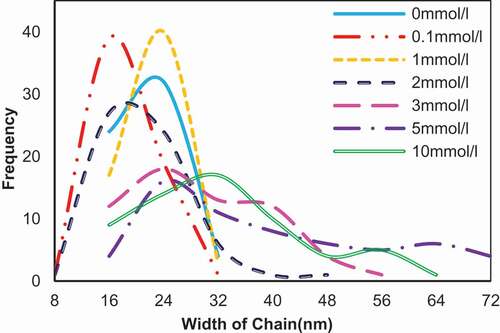
Figure 7. Width of the network chains (n = 60) of the self-assembly of 0.02 mg/ml SA in the presence of CaCl2 in different concentration. The ordinate refers to the diameter count in each interval.