Figures & data
Table 1. Physicochemical properties of RBIF
Table 2. In vitro maximum binding capacity (BCmax) of RBIDF for Pb2+ at pH 2.0 and 7.0
Figure 1. SEM images of exterior surface of modified rice bran fiber treated with 0.2%, 1.25%, and 2.0% H2SO4 combined with 1.25% KOH regimes
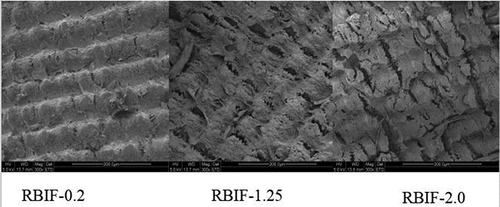
Table 3. Effect of Na and Ca on maximum binding capacity (BCmax) for Pb
Figure 2. Binding capacities of various RBIFs that produced with 0.2%, 1.25%, and 2.0% H2SO4 combined with 1.25% KOH for Pb2+ at different time
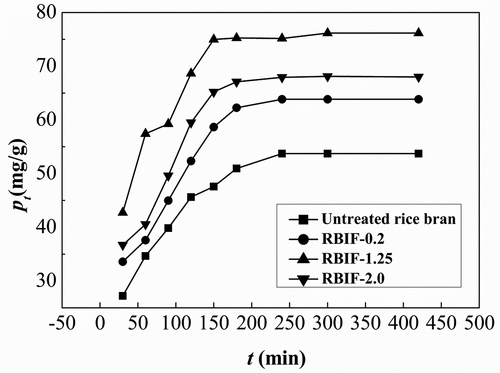
Table 4. Kinetic parameters for the adsorption of Pb by RBIF
Figure 3. The plot of intraparticle diffusion modeling of lead ion onto RBIF (C: 10 mmol/L; pH: 6.8)
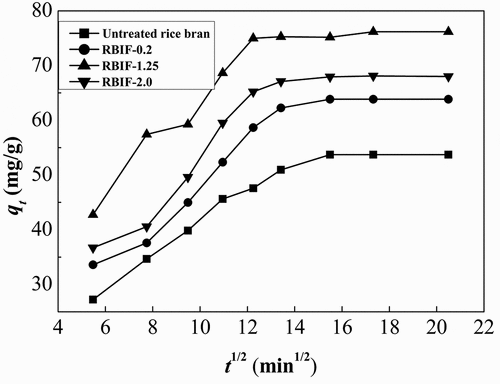
Table 5. Isotherm constants for Pb removal by RBIDF
Table 6. Calculated r values for Pb2+ sorption by RBIFs
Figure 4. Binding capacities of various RBIFs that produced with 0.2%, 1.25%, and 2.0% H2SO4 combined with 1.25% KOH for Pb2+ at different temperatures
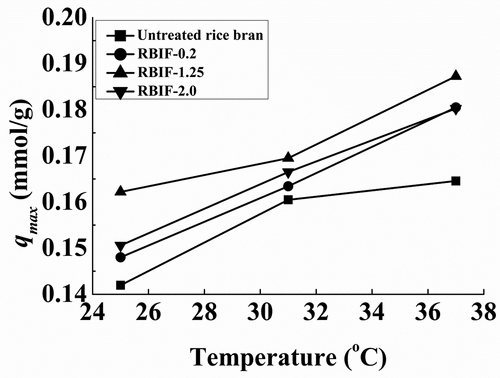
Table 7. Thermodynamic parameters for Pb2+ by RBIF
