Figures & data
Table 1. Calculated and observed masses for ETRF form the leaves of three Sonneratia species and possible monomeric composition
Figure 1. MALDI-TOF mass spectra of ETRE from the leaves of three Sonneratia species: S. apetala (a), S. caseolaris (b), and S. alba (c)
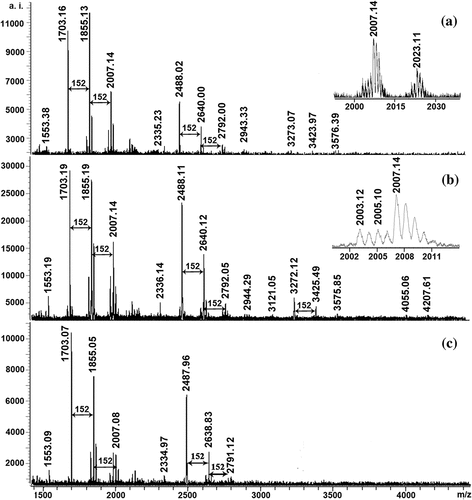
Figure 2. Reversed-phase HPLC chromatograms of ETRE from the leaves of three Sonneratia species after hydrolysis: S. apetala (a), S. caseolaris (b), and S. alba (c); Concentrations of total (d) and free (e) gallic acid and ellagic acid in hydrolyzed and unhydrolyzed ETRE from the leaves of three Sonneratia species. Peaks: 1, gallic acid; 2, ellagic acid; 3 and 4, unidentified compounds
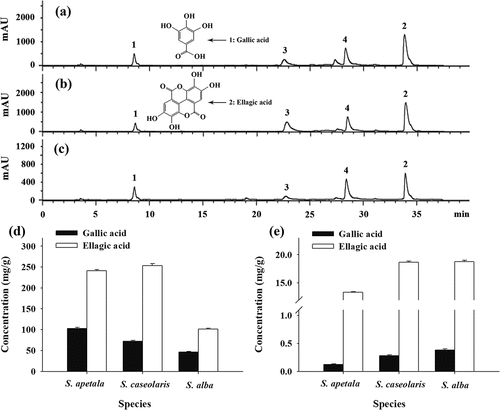
Figure 3. Antioxidant activities of ETRE from the leaves of three Sonneratia species determined by DPPH, ABTS, and FRAP assays. Different letters (a–c) above bars represent significant differences from each other at P < 0.05 level. Butylated hydroxyanisole (BHA) was used as a positive control
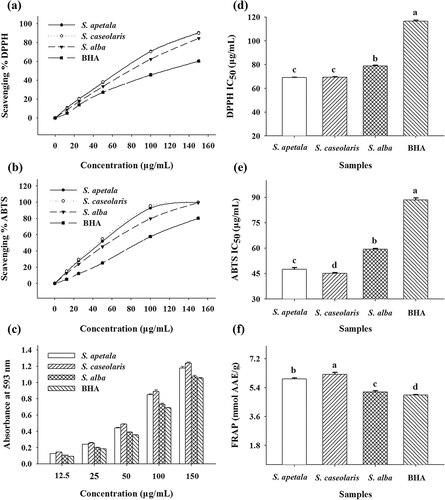
Figure 4. Inhibitory effects of ETRE from the leaves of three Sonneratia species on the activity of α-amylase. Acarbose was used as a positive control
Figure 5. (a–c) Fluorescence emission spectra of α-amylase in the absence and presence of ETRE from the leaves of three Sonneratia species at 290 k. The concentrations of ETRE for curves a-e were 0, 1.0, 2.0, 5.0, and 8.0 µg/mL, respectively; (d–f) Stern–Volmer plots for α-amylase quenching by ETRE at different temperatures; (g–i) Plots of log[(F0-F)/F] versus log[Q] for the binding of ETRE with α-amylase at different temperatures. [Q] and T represent the concentrations of ETRE and temperature, respectively
![Figure 5. (a–c) Fluorescence emission spectra of α-amylase in the absence and presence of ETRE from the leaves of three Sonneratia species at 290 k. The concentrations of ETRE for curves a-e were 0, 1.0, 2.0, 5.0, and 8.0 µg/mL, respectively; (d–f) Stern–Volmer plots for α-amylase quenching by ETRE at different temperatures; (g–i) Plots of log[(F0-F)/F] versus log[Q] for the binding of ETRE with α-amylase at different temperatures. [Q] and T represent the concentrations of ETRE and temperature, respectively](/cms/asset/d4cf9f4f-e856-435e-afba-671aa8081728/ljfp_a_1675693_f0005_oc.jpg)
Table 2. The quenching parameters of ETRF form the leaves of three Sonneratia species against α-amylase
