Figures & data
Table 1. Formulation of Hydrogels with and without Polyherbal extract.
Table 2. Percentage yield of n-hexane and methanol extract.
Table 3. pH of hydrogel formulations with and without Polyherbal extract.
Table 4. Appearance, homogeneity, spreadability, and extrudability of hydrogel formulations.
Figure 1. Rheological studies of hydrogel formulations with and without polyherbal extract (PNE, PME). Sample G is hydro-gel formulation without polyherbal extract, Sample MG 1% is hydrogel formulation containing 1.0 g of polyherbal methanolic extract, Sample MG 3% is hydrogel formulation containing 3.0 g of polyherbal methanolic extract and sample MG 5% is hydrogel formulation containing 5.0 g of polyherbal methanolic extract, Sample NG 1% is hydrogel formulation containing 1.0 g of polyherbal n-hexane extract, Sample NG 3% is hydrogel formulation containing 3.0 g of polyherbal n-hexane extract and sample NG 5% is hydrogel formulation containing 5.0 g of polyherbal n-hexane extract
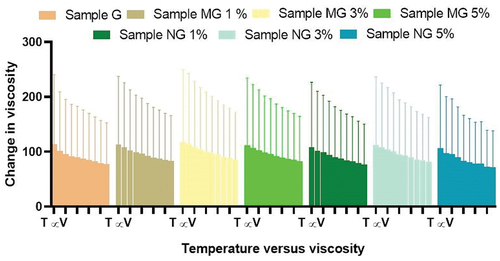
Table 5. Stability study of hydrogel formulations with and without different extracts.
Table 6. Skin irritation test of hydrogel formulations with and without polyherbal extract.
Figure 2. Analgesic activity of polyherbal n-hexane extracts (PNE) by tail flick method.
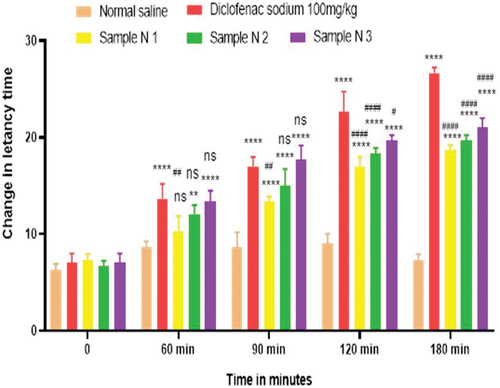
Figure 3. Analgesic activity of polyherbal methanolic extracts (PME) by tail flick method.
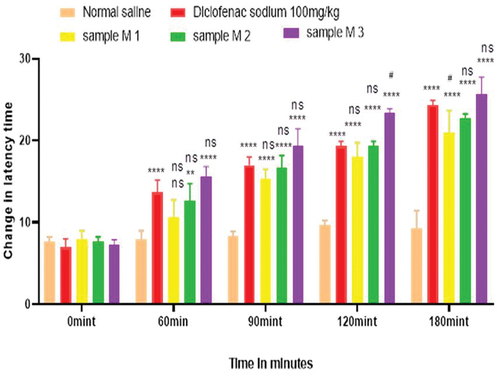
Figure 4. Analgesic activity of hydrogel formulations (NG) with polyherbal n-hexane extracts by tail flick method.
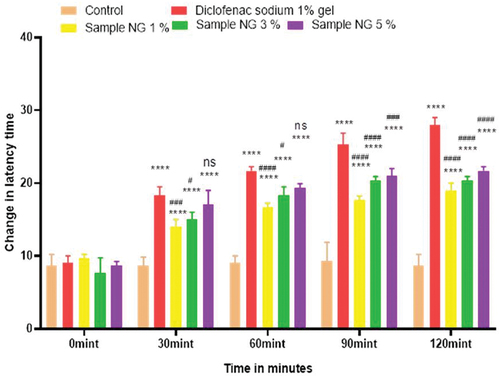
Figure 5. Analgesic activity of hydrogel formulations (MG) with polyherbal methanolic extracts by tail flick method.
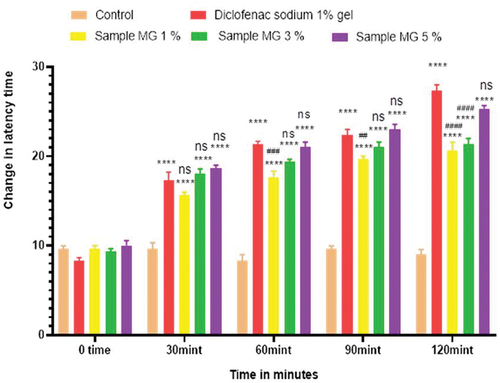
Figure 6. Analgesic activity of polyherbal n-hexane extracts (PNE) by hot plate method.
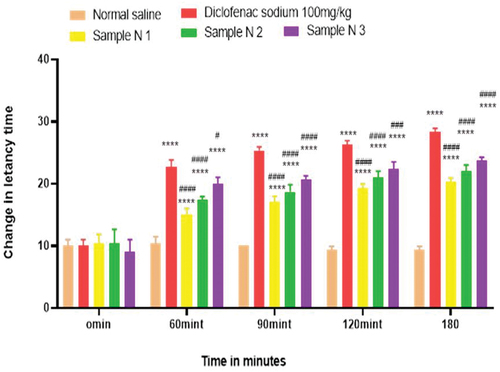
Figure 7. Analgesic activity of polyherbal methanolic extracts (PME) by hot plate method.
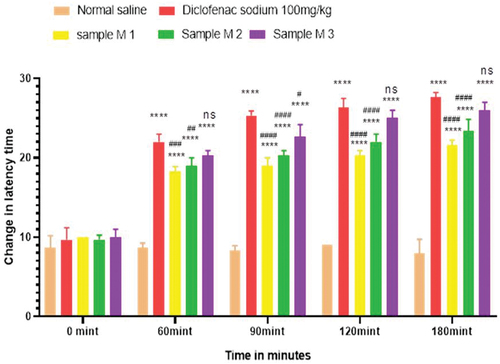
Figure 8. Analgesic activity of hydrogel formulations (NG) with polyherbal n-hexane extracts by hot plate method.
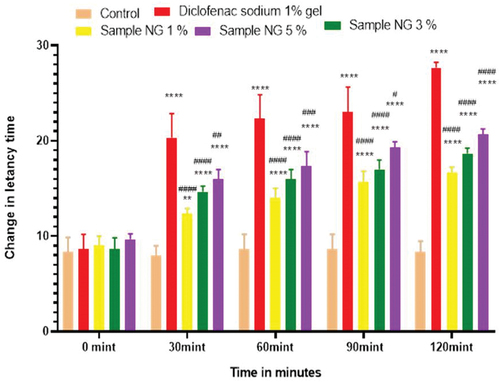
Figure 9. Analgesic activity of hydrogel formulations (MG) with polyherbal methanolic extracts by hot plate method.
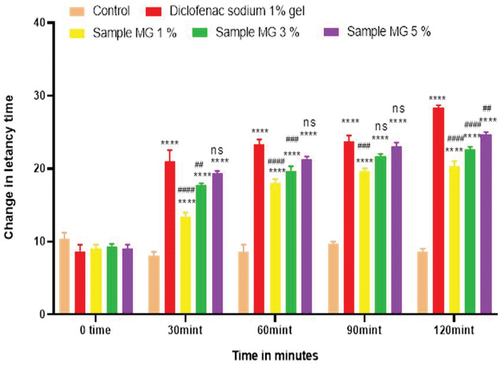
Figure 10. Anti-inflammatory activity of polyherbal n-hexane extracts (PNE).
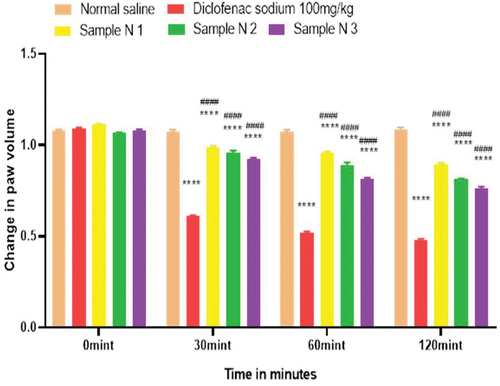
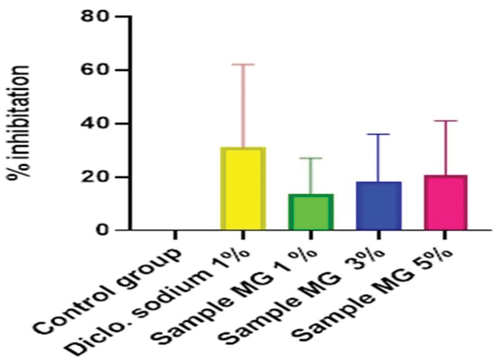
Figure 11. %inhibition of anti-inflammatory activity of polyherbaln-hexane extracts (PNE).
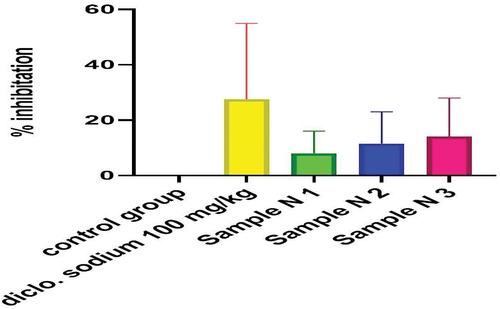
Figure 12. Anti-inflammatory activity of polyherbal methanolic extracts (PME).
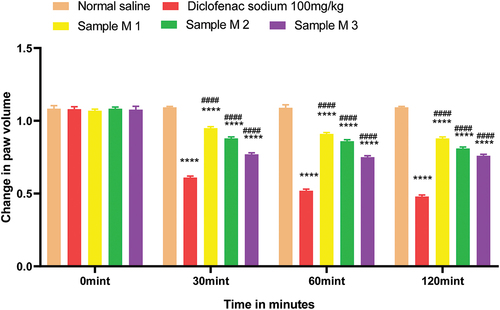
Figure 13. %inhibition of anti-inflammatory activity of polyherbal methanolic extracts (PME).
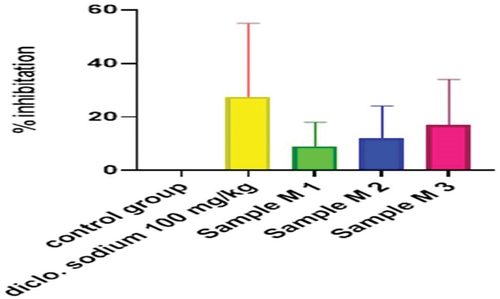
Figure 14. Anti-inflammatory activity of hydrogel formulations (NG) with polyherbal n-hexane extracts.
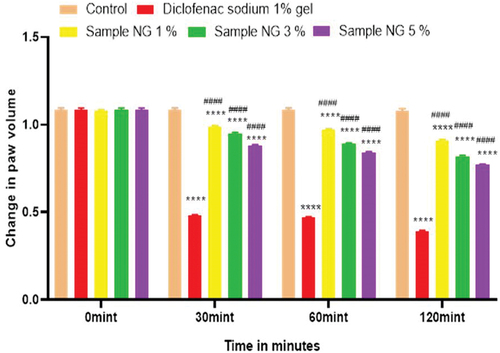
Figure 15. % inhibition of anti-inflammatory activity of hydrogel formulations (NG) with polyherbal n-hexane extracts.
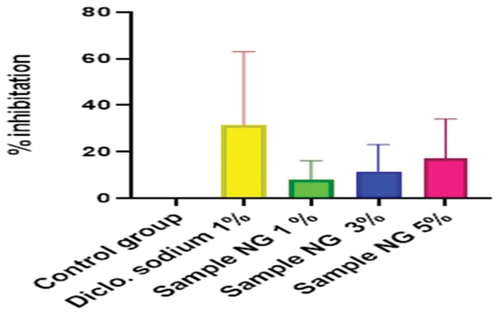
Figure 16. Anti-inflammatory activity of hydrogel formulations (MG) with polyherbal methanolic extracts.
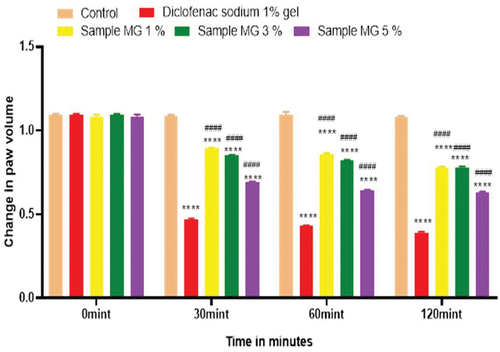
Figure 17. %inhibition of anti-inflammatory activity of hydrogel formulations(MG) with polyherbal methanolic extracts.