Figures & data
Figure 1. Diffusible metabolite assay in dual culture. Inhibition rate was calculated as [(Rc − Rexp)/Rc] × 100%, where Rc represents the longest diameter of the fungal mycelium and Rexp the horizontal diameter of the pathogenic fungus (which show the inhibitory effect).
![Figure 1. Diffusible metabolite assay in dual culture. Inhibition rate was calculated as [(Rc − Rexp)/Rc] × 100%, where Rc represents the longest diameter of the fungal mycelium and Rexp the horizontal diameter of the pathogenic fungus (which show the inhibitory effect).](/cms/asset/2b60f531-0665-42b8-af46-73457d0c0ffa/tmyb_a_1454013_f0001_c.jpg)
Figure 2. Detection of volatile compounds secreted from antagonistic yeasts according to the ‘‘mouth-to-mouth” method. To study the efficacy of selected antagonist on the growth inhibition of pathogenic fungus, radial mycelia growth in two vertical directions randomly chosen was measured (Rexp and Rc, which represents the diameter of fungal mycelium with yeast (upper panel) and in the absence of yeast (control, lower panel), respectively). Inhibition rate was calculated as [(Rc2 − Rexp2)/Rc2] × 100%.
![Figure 2. Detection of volatile compounds secreted from antagonistic yeasts according to the ‘‘mouth-to-mouth” method. To study the efficacy of selected antagonist on the growth inhibition of pathogenic fungus, radial mycelia growth in two vertical directions randomly chosen was measured (Rexp and Rc, which represents the diameter of fungal mycelium with yeast (upper panel) and in the absence of yeast (control, lower panel), respectively). Inhibition rate was calculated as [(Rc2 − Rexp2)/Rc2] × 100%.](/cms/asset/094e1677-4f00-44f0-adc3-a2aff497cce0/tmyb_a_1454013_f0002_c.jpg)
Figure 3. Detection of hydrolytic enzymes activity of antagonistic yeasts based on their reactions in a culture medium containing the substrate of the candidate enzymes. (A) Extracellular chitinase activity was detected on colloidal chitin media containing bromocresol purple (pH 4.7). (B) Detection of protease activity of antagonistic yeasts based on culturing yeast in a culture medium containing casein. (C) CMCase activity was detected on carboxymethylcellulose media. (D) Comparison of protease and CMCase was based on the following ratio: R/r, where R is the diameter of the entire clear zone and is the diameter of the zone with yeast colonies.
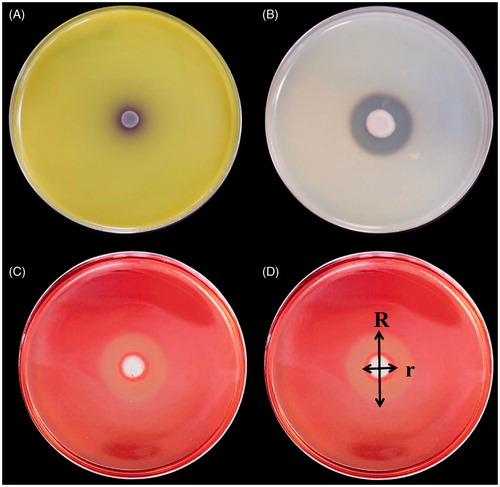
Figure 4. Efficacy of the volatiles produced by antagonistic yeast in postharvest control of B. cinerea on strawberry. Yeast cultures and strawberries with B. cinerea spores on the surface were placed into a sealed plastic box at high relative humidity to ensure favorable conditions for the postharvest onset of the disease.
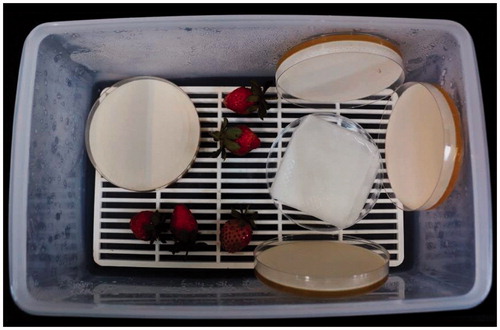
Figure 5. Evaluation of in vitro antagonism by production of diffusible substances by yeasts against the B. cinerea JYC2142 on YPD plate. (A) B. cinerea alone as the control group. (B) Yeasts (right) were antagonistic to B. cinerea (left). (C) Yeasts (right) were not antagonistic to B. cinerea (left).
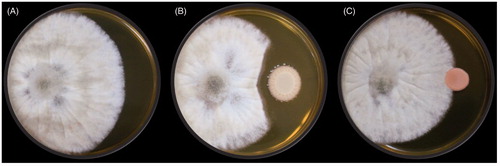
Table 1. Antagonistic yeasts with the accession numbers of large subunit (LSU) of ribosomal DNA sequences.
Table 2. In vitro antagonistic activity assays of antagonistic yeast.
Table 3. Pathogens of strawberry with the accession numbers of ITS1-5.8S-ITS2 and partial large subunit (LSU) of ribosomal DNA sequences.
Table 4. In vitro antagonistic activity assays of antagonistic yeast against other pathogenic fungi by diffusible compounds.
Table 5. In vitro antagonistic activity assays of antagonistic yeast against other pathogenic fungi by volatile compounds.
Figure 6. Extracellular chitinase activity was detected on colloidal chitin media containing bromocresol purple (pH 4.7). Three antagonistic yeast (JYC1146, JYC1278, and JYC1291) that had best ability of volatile inhibition and other six strains (JYC1525, JYC137, JYC2120, JYC138, JYC2041, and JYC1061) that had ability of diffusible inhibition to test if they had biofilm-forming capacity. Ability evaluated as the concentration of crystal violet solubilizing from the retained yeast adhered to the well.
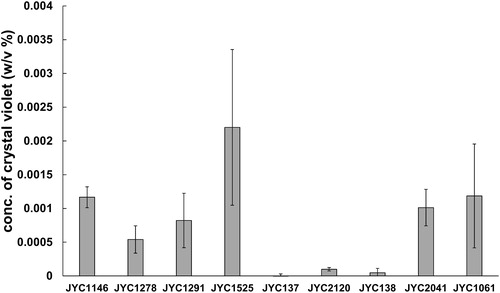
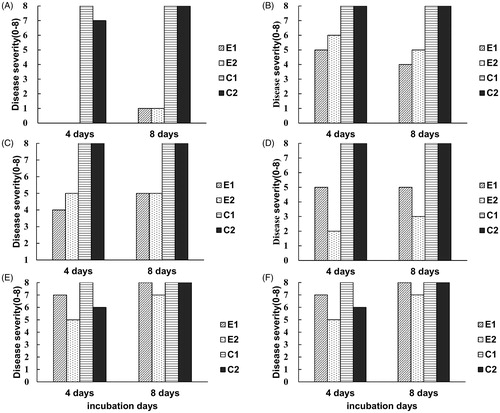
Figure 7. Biocontrol efficacy of the three selected antagonistic yeasts that had best ability of volatile inhibition on the decay of strawberries caused by B. cinerea. (A–C) evaluating the efficacy of inhibition of gray mold infection on strawberries by four petri dishes with strain Galactomyces candidum JYC1146, Aureobasidium pullulans JYC1278, and A. pullulans JYC1291 cultures, respectively. (D–F) evaluating the efficacy of inhibition of gray mold infection on strawberries by two petri dishes with strain Gal. candidum JYC1146, A. pullulans JYC1278 and A. pullulans JYC1291 cultures, respectively. E1 and E2 represents two repeats of the experiment set with yeast treatment. C1 and C2 represents two repeats of the experiment set without yeast treatment.