Figures & data
Figure 1. Multiple alignments of representative β-galactosidases from thermophiles.

Figure 2. Purification of the recombinant β-galactosidase.
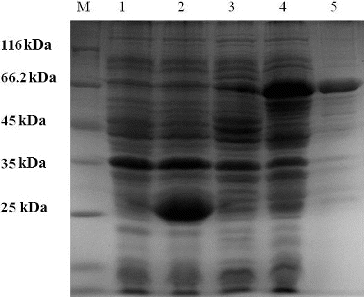
Figure 3. Effects of temperature on recombinant β-galactosidase activity (A) and stability (B).
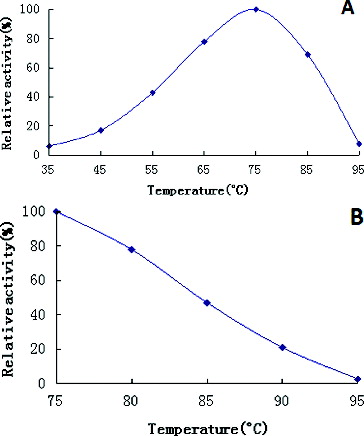
Figure 4. Effects of pH on β-galactosidase activity (A) and stability (B).
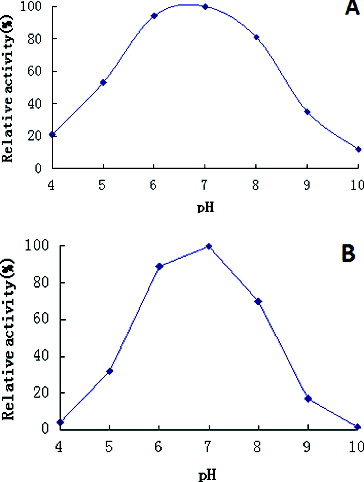
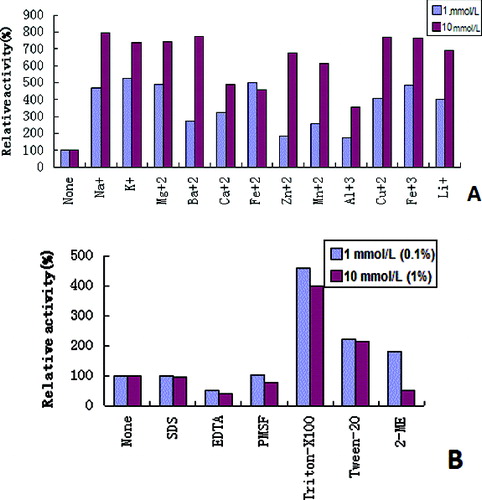
Figure 5. Effects of metal ions (A) and additives, and detergents (B) on β-galactosidase activity.