Figures & data
Table 1. GC-MS data of the volatiles of the extracts of Arum maculatum roots.
Figure 1. Antiangiogenic effects of A. maculatum extracts against EA.hy926 cells after 72 h. exposure (МТТ-assay).
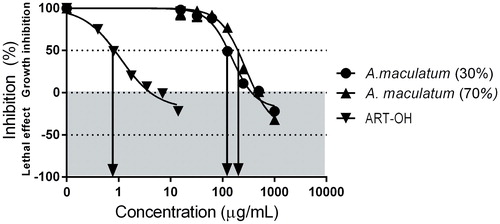
Table 2. Antiproliferative effects of the tested A. maculatum extracts against EA.hy926 vascular endothelial cells (MTT-dye reduction assay).
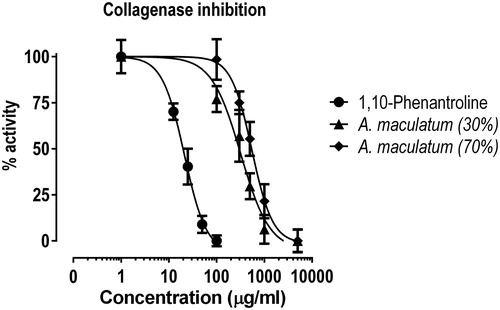
Table 3. Inhibitory activity of the tested extracts on bacterial collagenase.
Table 4. Inhibitory effects of the tested extracts on cyclooxygenase (COX-1 and COX-2) activities.
Figure 3. Effects of A. maculatum hydro-alcoholic extracts on PHA/PMA-induced secretion of IL-2 in Jurkat E.6 cells (human Т-cell model).
Note: * Indicates statistical significance at p < 0.05 vs. PHA + PMA alone; # indicates significant difference vs. the 70% EtOH extract; paired t-test.
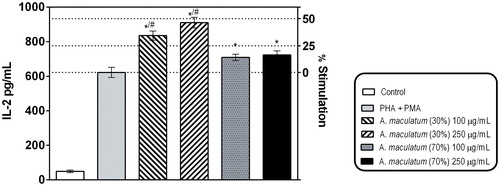
Figure 4. Concentration dependence of the anti-radical activity of the 30% EtOH extract determined in stable free radical-based systems.
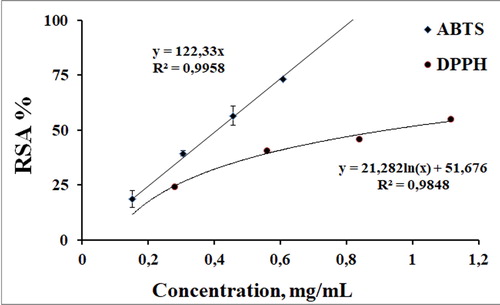
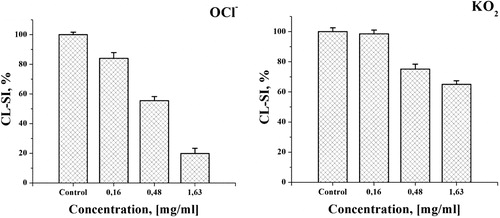
Figure 5. Typical chemiluminescent curves obtained in the chemilumiescent model systems used for evaluation of the superoxide anion and hypochlorte scavenging activity.
Note: X-axis, time of analysis [s]; Y-axis, intensity of chemiluminescence signal - relative units [RU]. Blank samples contained PBS, the tested antioxidants, and the reaction-intitiating agents; control samples contained PBS, luminol and the reaction-initiating agents but without the tested antioxidants.
![Figure 5. Typical chemiluminescent curves obtained in the chemilumiescent model systems used for evaluation of the superoxide anion and hypochlorte scavenging activity.Note: X-axis, time of analysis [s]; Y-axis, intensity of chemiluminescence signal - relative units [RU]. Blank samples contained PBS, the tested antioxidants, and the reaction-intitiating agents; control samples contained PBS, luminol and the reaction-initiating agents but without the tested antioxidants.](/cms/asset/18816f31-5b16-4da5-966b-32ec8efb27ec/tbeq_a_1722239_f0005_b.jpg)