Figures & data
Figure 1. A sample of the Escherichia coli thiol-redox network showing the kinetic and spatial separation of electron flow pathways. Within the cytoplasm, reducing equivalents from NADPH and NADH are used by the reductases (circles), thioredoxin reductase (TrxB), glutathione reductase (Gor) and alkyl hydroperoxide reductase subunit F (AhpF) to reduce thioredoxin (Trx), oxidized glutathione and alkyl hydroperoxidase subunit C (AhpC) respectively. In turn, other targets such as glutaredoxins (Grx), ribonucleotide reductase (Nrd), thiol-peroxidase (Tpx) and the transcription factor OxyR, are reduced by thiol-disulfide exchange [Citation6,Citation7]. Within the periplasmic space, protein thiols are oxidized by DsbA which in turn is oxidized by DsbB [Citation8]. Arrows show the electron flow pathways between cognate redox partners. The redox potentials for GrxA, TrxA and DsbA were obtained from Ref [Citation9]; the redox potentials for E. coli AhpC and Tpx were assumed to be similar to Salmonella typhimurium AhpC [Citation10], while the redox potential for DsbB was also assumed to be a midway between the isolated DsbB and ubiquinone [Citation11]. The hypothetical distribution between the oxidized (pink) and reduced (blue) moieties are shown for each redox couple with the NADPH and NADH electron sources shown in blue and, the hydrogen peroxide and ribonucleotide reductase electron sinks shown in pink.
![Figure 1. A sample of the Escherichia coli thiol-redox network showing the kinetic and spatial separation of electron flow pathways. Within the cytoplasm, reducing equivalents from NADPH and NADH are used by the reductases (circles), thioredoxin reductase (TrxB), glutathione reductase (Gor) and alkyl hydroperoxide reductase subunit F (AhpF) to reduce thioredoxin (Trx), oxidized glutathione and alkyl hydroperoxidase subunit C (AhpC) respectively. In turn, other targets such as glutaredoxins (Grx), ribonucleotide reductase (Nrd), thiol-peroxidase (Tpx) and the transcription factor OxyR, are reduced by thiol-disulfide exchange [Citation6,Citation7]. Within the periplasmic space, protein thiols are oxidized by DsbA which in turn is oxidized by DsbB [Citation8]. Arrows show the electron flow pathways between cognate redox partners. The redox potentials for GrxA, TrxA and DsbA were obtained from Ref [Citation9]; the redox potentials for E. coli AhpC and Tpx were assumed to be similar to Salmonella typhimurium AhpC [Citation10], while the redox potential for DsbB was also assumed to be a midway between the isolated DsbB and ubiquinone [Citation11]. The hypothetical distribution between the oxidized (pink) and reduced (blue) moieties are shown for each redox couple with the NADPH and NADH electron sources shown in blue and, the hydrogen peroxide and ribonucleotide reductase electron sinks shown in pink.](/cms/asset/4ba7eb4f-7fde-4366-9028-60bfc20206df/yrer_a_1966183_f0001_oc.jpg)
Table 1. Examples of synthetic biology applications that utilize thiol-based components
Figure 2. OxyR is used as a specific hydrogen peroxide sensor in genetic circuits (A) Genetic comparator circuit built using an OxyR part. In this circuit, OxyR activation by hydrogen peroxide results in the expression of the recombinase Bxb1 which recognizes a computationally designed ribosome binding site (RBS). Recombination of these sites leads to GFP expression [Citation165]. By adjusting the ribosome binding sequences, promoter (↱) and terminator (Т) sequences, the GFP-output of this part could be varied. In (B), OxyR was used to induce the transcription of the CheZ phosphatase which dephosphorylated CheY which subsequently reduced E. coli tumbling [Citation166].
![Figure 2. OxyR is used as a specific hydrogen peroxide sensor in genetic circuits (A) Genetic comparator circuit built using an OxyR part. In this circuit, OxyR activation by hydrogen peroxide results in the expression of the recombinase Bxb1 which recognizes a computationally designed ribosome binding site (RBS). Recombination of these sites leads to GFP expression [Citation165]. By adjusting the ribosome binding sequences, promoter (↱) and terminator (Т) sequences, the GFP-output of this part could be varied. In (B), OxyR was used to induce the transcription of the CheZ phosphatase which dephosphorylated CheY which subsequently reduced E. coli tumbling [Citation166].](/cms/asset/5340a36c-7448-461e-a28f-d5f6e0fe671b/yrer_a_1966183_f0002_oc.jpg)
Figure 3. Electron flow pathways within E. coli SHuffle2 cells that support cytoplasmic disulfide bond formation. In these cells, thioredoxin and glutathione reductase have been deleted (cf. ), and a mutant peroxidase (AhpC*) reduced glutathionylated glutaredoxin and GSH [Citation92] to support metabolic pathways such as ribonucleotide reductase (Nrd) cycling. A PDI-GPx7 chimera was used to reduce hydrogen peroxide and, together with thioredoxin, oxidize protein thiols, while disulfide isomerization by DsbC was used to enable correctly folding of a target antibody [Citation176]. The hypothetical distribution between the oxidized (pink) and reduced (blue) moieties are shown for each redox couple.
![Figure 3. Electron flow pathways within E. coli SHuffle2 cells that support cytoplasmic disulfide bond formation. In these cells, thioredoxin and glutathione reductase have been deleted (cf. Figure 1), and a mutant peroxidase (AhpC*) reduced glutathionylated glutaredoxin and GSH [Citation92] to support metabolic pathways such as ribonucleotide reductase (Nrd) cycling. A PDI-GPx7 chimera was used to reduce hydrogen peroxide and, together with thioredoxin, oxidize protein thiols, while disulfide isomerization by DsbC was used to enable correctly folding of a target antibody [Citation176]. The hypothetical distribution between the oxidized (pink) and reduced (blue) moieties are shown for each redox couple.](/cms/asset/72c72e8a-dbc4-4e85-a66d-c1e381f6e510/yrer_a_1966183_f0003_oc.jpg)
Figure 4. Genetically encoded redox sensors have been developed using circularly permuted fluorescent proteins (A) or redox-sensitive fluorescent proteins (B). Circularly permuted redox proteins contain a redox domain that can bind specific redox species which perturbs their fluorescent output. In contrast, redox-active fluorescent proteins contain a flexible linker region on either the C- or N- terminus and, a redoxin protein (RX) that transfers redox equivalents to redox-sensitive cysteines on the fluorescent protein, affecting probe fluorescence.
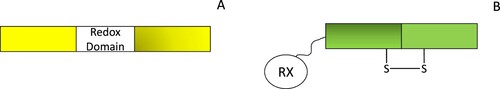
Table 2. Computational models of redoxin systems.
