Figures & data
Figure 1. Disposition of study subjects.
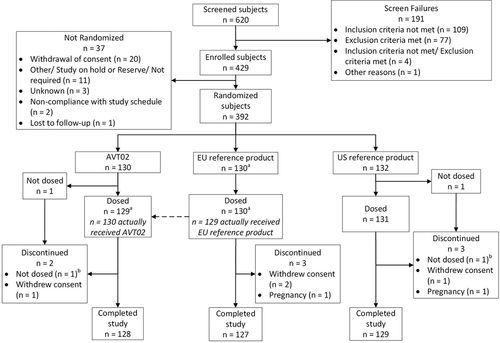
Table 1. Demographic and baseline characteristics (full analysis set)
Figure 2. Mean serum adalimumab concentrations over time by treatment (semi log; pharmacokinetic population).
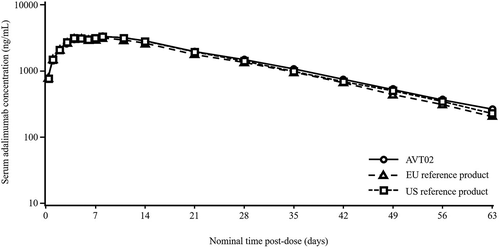
Table 2. Summary of serum pharmacokinetic parameters for adalimumab by treatment (pharmacokinetic population)
Table 3. Overview of bioequivalence assessment of adalimumab primary pharmacokinetic parameters (pharmacokinetic population)
Table 4. Treatment-emergent adverse events (safety set)
Table 5. Summary of immunogenicity (immunogenicity population)
