Figures & data
Table 1. Literature search terms.
Table 2. Inclusion and exclusion criteria for the analysis.
Figure 1. PRISMA diagram for the systematic literature review of articles that evaluated the treatment of previously treated adult human subjects diagnosed with moderate-to-severe hemophilia (FIX levels ≤2 %) receiving rFIX either prophylactically or on-demand. aSome articles met more than one criterion. bNine articles met the inclusion criteria initially, but Solano Trujillo et al.Citation17 and Oldenburg et al.Citation14 were excluded from further analysis as they included duplicate patients from Windyga et al.Citation16 and Collins et al.Citation10, respectively. cOf the remaining seven articles, Collins et al.Citation10 was excluded from the statistical analysis as it did not include mean/SD data for analysis. dIneligible articles consisted of manuscripts which did not describe results of a Phase III study, including post-hoc analyses, meta-analyses, and post-marketing surveillance. eExclusions for non-human subjects included any study using animals or animal tissues or in vivo, in vitro, or ex vivo studies. fIneligible study types were those that were not Phase III clinical trials, including surgical sub-studies and extension studies. gAny study where the patients were not diagnosed with hemophilia B were excluded. hStudies not treating participants with a recombinant FIX product were excluded. iEligible studies must include outcomes from at least one of the following; ABR, AsBR, AjBR. jPediatric studies were excluded, including those who combined adult and pediatric data.
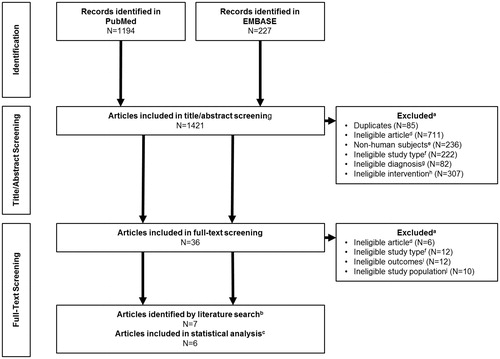
Table 3. Study characteristics for included articles.
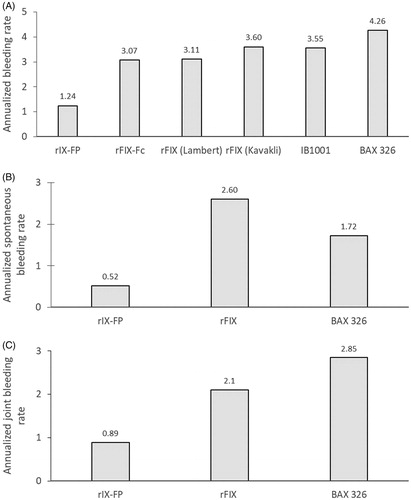
Table 4. Details of bleeding rates of prophylaxis patients in rFIX studies identified by the systematic review.
Table 5. Summary of statistical analysis results.