Figures & data
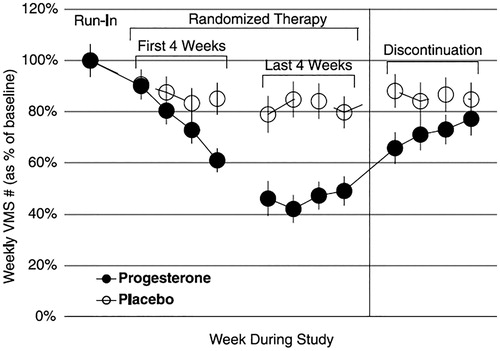
Figure 1. The four lines on the Daily Menopause Diary© used to describe day and night vasomotor symptoms (A) and a typical symptomatic woman’s record over a week (B). The data from B can be used to create a VMS Score of 107 = daytime # × intensity = 80 + nighttime # × intensity = 27; the number of moderate-intense VMS #/week = 45 (falling short of criteria for ‘severe VMS’; Night wakening with VMS = 11/week.
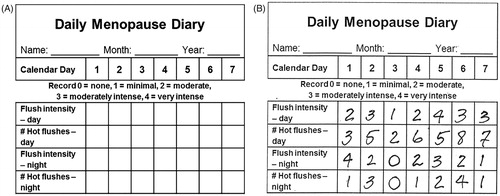
Figure 2. Data from randomized clinical trial on oral micronized progesterone (Progesterone) or placebo at the end of the study reported their change (from −5 to +5) by therapy assignment, shown here in box-and-whiskers plots of ratings for (a) changes in daytime hot flush number, (b) changes in daytime hot flush intensity (severity), (c) changes in night sweat frequency, (d) changes in night sweat intensity, and (e) changes in sleep quality. All differences are significant by Mann–Whitney U-test in favor of progesterone (p = 0.001 to 0.013). (Reprinted with permission from MenopauseCitation55).
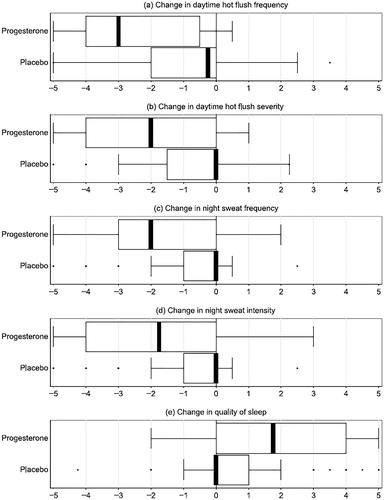
Figure 3. The percentage change from baseline (run-in) in the number of day and night hot flushes per week in the first and third of 4 weeks of 12-week treatment on oral micronized progesterone (Progesterone) and 4 weeks after stopping (dark circles) or placebo (open circles) therapy with SEM error bars. Ninety-five percent confidence intervals of the difference between the discontinuation phase and the run-in showed that, on progesterone, both VMS Score (−1.3, −9.9) and VMS daily number (−0.1 to −3.0) remained less than their baseline. By contrast, at 4 weeks of discontinuation, those assigned to placebo did not differ from their baseline in VMS daily number or in VMS Score. With permission from Gynecological EndocrinologyCitation58.