Figures & data
Figure 1 (a). (A) Lineweaver-Burk plot for the inhibition of xanthine oxidase by 6-(N-benzoylamino)purine. Enzyme concentration used was 2.25 units/ml. Xanthine concentration was varied (0.30 μM, 0.50 μM, 0.80 μM, 1.00 μM, 2.00 μM) at a series of fixed concentrations of 6-(N-benzoylamino)purine. (B) Inhibition type and Kii value of 6-(N-benzoylamino)purine. (b). Time course of xanthine oxidase-catalyzed reaction in the absence and presence of allopurinol and 6-(N-benzoylamino)purine. 1) 0 μM inhibitor, 2) 2.5 μM allopurinol, 3) 5 μM allopurinol, 4) 2.5 μM 6-(N-benzoylamino)purine, 5) 5 μM 6-(N-benzoylamino)purine, 6) 7.5 μM allopurinol, 7) 7.5 μM 6-(N-benzoylamino)purine. Enzyme concentration used was 2.25 units/ml. Level of enzymatically generated uric acid formation was measured at 293 nm.
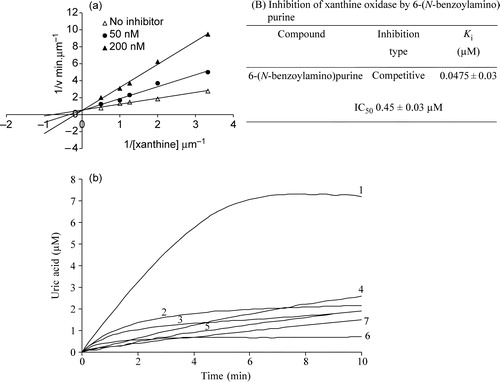
Table I. Percentage inhibition of XO in the presence of increasing concentrations of xanthine and 10 μM of 6-(N-benzoylamino)purine. In Set1 experiments, 2.25 units of XO was added to 1 ml of 100 mM Sodium phosphate buffer, pH 7.5 containing different concentrations of xanthine (10–100 μM) in presence and absence of inhibitor. In set 2 experiments, 2.25 units of XO and 10 μM of 6-(N-benzoylamino)purine was incubated and added to 1 ml sodium phosphate buffer containing different concentrations of xanthine as indicated.
Figure 2 (a). Xanthine, allopurinol and 6-(N-benzoylamino)purine induced enzymatic electron transfer reaction. The reaction mixture contained 100 mM sodium phosphate, pH 7.4, 10 μM xanthine or 10 μM allopurinol or 10 μM 6-(N-benzoylamino)purine and 15 μM DCPIP. The reaction was initiated with the addition of 2.25 units of xanthine oxidase and the decrease in absorbance with time was measured at 605 nm. (b). Xanthine, allopurinol and 6-(N-benzoylamino)purine induced enzymatic superoxide formation. The reaction mixture contained 100 mM sodium phosphate, pH 7.4, 10 μM xanthine or 10 μM allopurinol or 10 μM 6-(N-benzoylamino)purine and 15 μM Cyt c. The reaction was initiated with the addition of 2.25 units of xanthine oxidase and the increase in absorbance with time was measured at 550 nm.
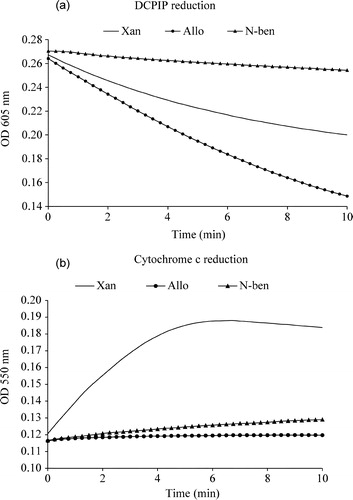
Figure 3 Binding mode of 6-(N-benzoylamino)purine in the active site of bovine milk XO. The hydrogen bonding interactions are shown as broken lines. Purine nucleus is coloured in blue and green. The nitrogen atoms of the purine are labeled in blue colour. Benzoyl group of 6-(N-Benzoylamino)purine is labelled in green and oxygen moiety in general (both for the inhibitor and the enzyme) is shown in red colour.
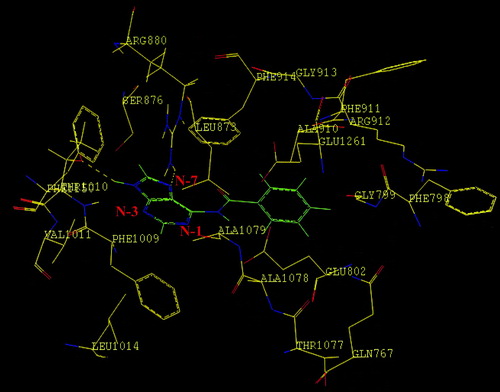
Table II. The IC50, Ki and binding energy values of adenine, 8-bromoxanthine and 6-(N-benzoylamino)purine with XO.