Figures & data
Figure 1. Alignment of the amino acid sequence of isoform CA III with that of isozymes CA I and II (CA I numbering system used). Thirty-six active site residues previously defined as forming the active site [Citation27] are indicated by a mixture of asterisk, “plus” and “z” signs above the CA I sequence. Seventeen residues known to participate in a network of hydrogen bonds and being involved in the binding of inhibitors/activators [Citation28] are indicated by “plus” and “z” above the sequence; the latter sign indicates the three zinc-liganded histidine residues (His94, 96 and 119). Conserved amino acids in the three isoforms are indicated by a closed box.
![Figure 1. Alignment of the amino acid sequence of isoform CA III with that of isozymes CA I and II (CA I numbering system used). Thirty-six active site residues previously defined as forming the active site [Citation27] are indicated by a mixture of asterisk, “plus” and “z” signs above the CA I sequence. Seventeen residues known to participate in a network of hydrogen bonds and being involved in the binding of inhibitors/activators [Citation28] are indicated by “plus” and “z” above the sequence; the latter sign indicates the three zinc-liganded histidine residues (His94, 96 and 119). Conserved amino acids in the three isoforms are indicated by a closed box.](/cms/asset/03b7a2a9-37ac-43f3-9a97-b76334008966/ienz_a_290880_f0001_b.gif)
Table I. Kinetic parameters for the CO2 hydration reaction catalysed by the recombinant cytosolic hCA isozymes I-III, at 20°C and pH 7.5, and their inhibition data with acetazolamide AAZ(5-acetamido-1,3,4-thiadiazole-2-sulfonamide), a clinically used drug [Citation1].
Figure 2. SDS PAGE for the hCA III–GST fusion protein. The band of the fusion protein (with a molecular weight of around 50 kDa) appears only after addition of IPTG to the growth medium.
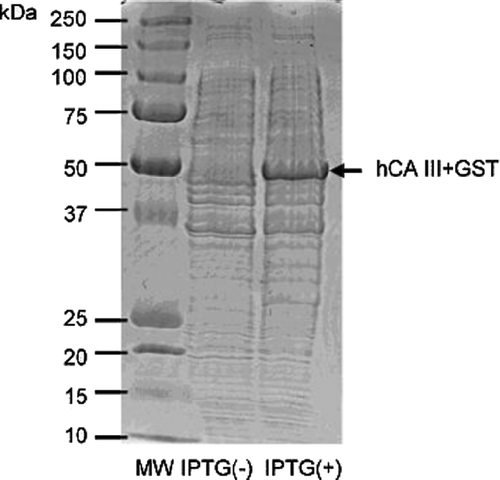
Figure 3. SDS PAGE for the hCA I- III proteins. Lanes: 1 = Ladder; 2 = hCA I; 3 = hCA II; 4 = hCA III. hCA I and II were from Sigma-Aldrich, whereas hCA III is the recombinant protein prepared by the GST fusion method, after the final purification steps.
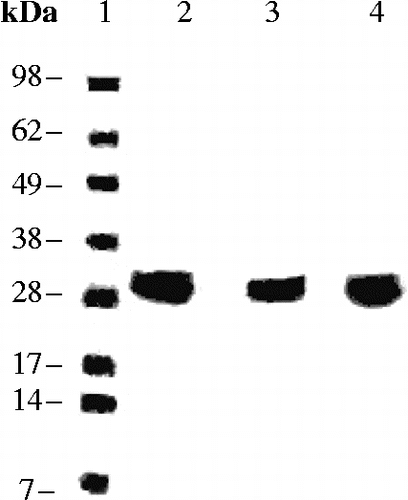
Table II. Inhibition of recombinant isozymes hCA I, II and III with anions by a stopped-flow kinetic assay monitoring the CO2 hydration reaction, at 20°C and pH 7.5 [Citation26].