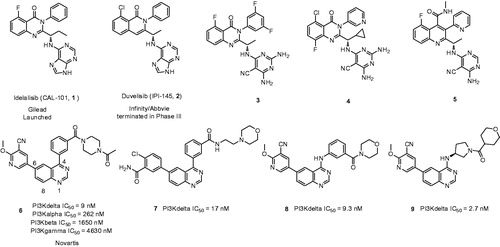
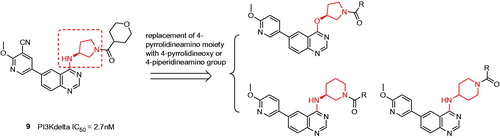
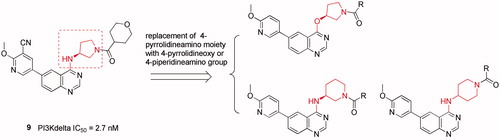
Figures & data
Scheme 1. Reagents and conditions: (a) (S)-1-Boc-3-hydroxypyrrolidine, anhydrous THF, NaH, rt, overnight, 70%; (b) 6-methoxy-3-pyridinylboronic acid, Na2CO3, PdCl2 (dppf), DME/H2O, reflux, 4 h, 65–81%; (c) (i)TFA, CH2Cl2, rt, 2 h, 23–91%; (ii) diverse acids, DMF, HATU, DIPEA, rt, 12 h, 23–91%; (d) (S)-1-Boc-3-aminopiperidine, DMF, DIPEA, 90 °C, 6 h, 90%; (e) 1-Boc-4-aminopiperidine, DMF, DIPEA, 90 °C, 6 h, 52%.
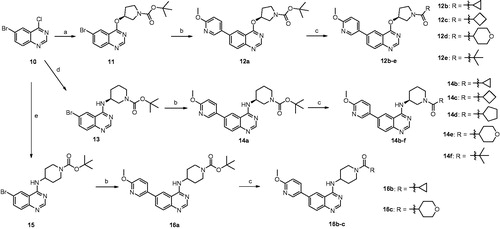
Table 1. PI3Kδ inhibitory activity of 4-pyrrolidineoxy substituted quinazolinesTable Footnotea.
Table 2. PI3Kδ inhibitory activity of 4-piperidineamino substituted quinazolinesTable Footnotea.
Table 3. Isoform selectivity of compounds against PI3K (p110α, p110β, p110γ, and p110δ)
Table 4. Anti-proliferative activities of new compounds in vitro