Figures & data
Scheme 1. Synthesis of the compounds 1–8. Ar: Phenyl (1); 4-methylphenyl (2); 4-Methoxyphenyl (3); 4-trifluoromethylphenyl (4); 3-hyrdoxyphenyl (5); 4-isopropylphenyl (6); 4-dimethylaminophenyl (7); 4-benzyloxyphenyl (8).
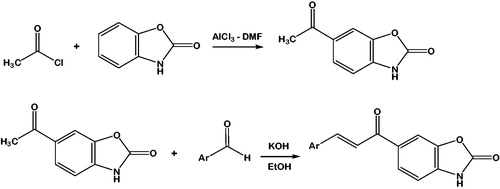
Figure 1. The details of 1H-NMR spectra of compound 2 as represantative 1H-NMR. δ (ppm) Ha: 8.05 (dd, 1H, JHa-Hb: 8.1 Hz, JHa-Hc: 1.5 Hz), Hb: 7.22 (d, 1H, JHa-Hb: 8.1 Hz), Hc: 8.08 (d, 1H, JHa-Hc: 1.5 Hz), Hd: 7.79 (d, 2H, JHd-He: 8.0 Hz), He: 7.27 (d, 2H, JHd-He: 8.0 Hz), Hα: 7.93 (d, 1H, JHα-Hβ: 15.5 Hz), Hβ: 7.70 (d, 1H, JHα-Hβ: 15.5 Hz) CH3 protons: 2.35 (s, 3H).
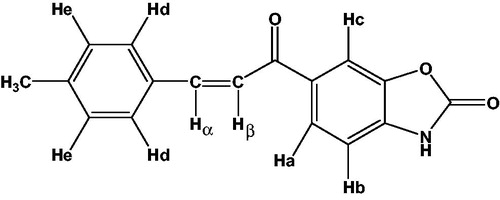
Table 1. Cytotoxic activities of the compounds 1–8 towards human OSCC cell lines and human oral normal cells
Table 2. Inhibitory effects of the compounds 1–8 on hCA I and hCA II isoenzymes.
