Figures & data
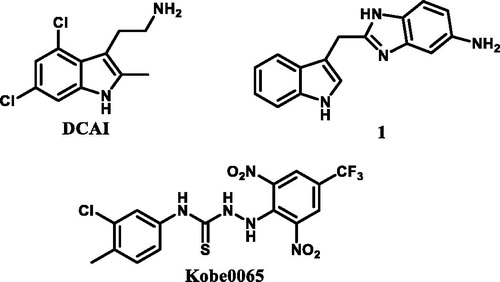
Scheme 1. (a) MeONa, MeOH, rt; (b) NH2NH2, MeOH; (c) CS2, TEA, THF, rt; (d) Boc2O, DMAP, 5 °C to rt; (e) C, CH3CN, TEA, rt. R1 = (o-tolyl)/(p-tolyl)/(3-chlorophenyl)/(2-chlorophenyl)/(4-(trifluoromethyl)phenyl)/(naphthalen-1-yl)/(3,5-dimethylphenyl); R2 = (2,6-dinitro-4-(trifluoromethyl)phenyl).
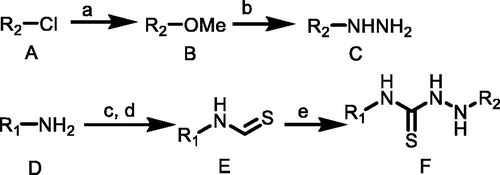
Scheme 2. (f) BTC, TEA, EA, 50 °C; (g) C, CH3CN, TEA, rt. R1 = 4-(trifluoromethyl)phenyl)/(3-chloro-4-methylphenyl); R2 = (2,6-dinitro-4-(trifluoromethyl)phenyl).
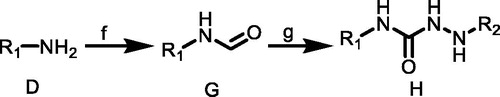
Scheme 3. (h) G, CH3CN, TEA, rt; (i) E, CH3CN, TEA, rt. R1 = 4-(trifluoromethyl)phenyl)/(3-chloro-4-methylphenyl); R2 = mesitylene/4-(trifluoromethyl)phenyl)/(1,1′-biphenyl)/(3s,5s,7s)-adamantan-1-yl)/(4-phenoxyphenyl).
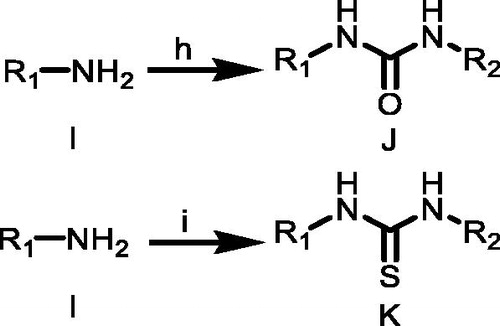
Scheme 4. (j) DCM, TEA, 1–5 °C; (k) HCl, H2O, CH3COOH, 1–5 °C; (l) NaNO2/H2O, 1–5 °C. R3 = 4-(trifluoromethyl)phenyl)/(3-chloro-4-methylphenyl).
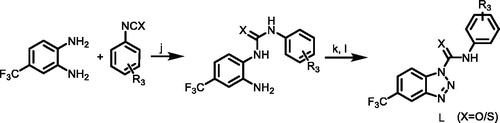
Table 1. Compounds TKR01-TKR07 and their anti-tumour activity.
Table 2. Compounds TKR08-TKR09 and their anti-tumour activity.
Table 3. Compounds TKR10–TKR18 and their anti-tumour activity.
Table 4. Compounds TKR19–TKR21 and their anti-tumour activity.
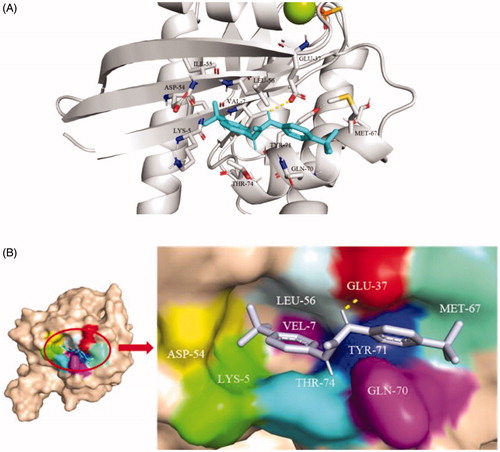
Figure 2. (A) Cartoon structure and (B) Surface structure of molecular docking analysis of small molecule TKR15 and K-RasG12V protein.