Figures & data
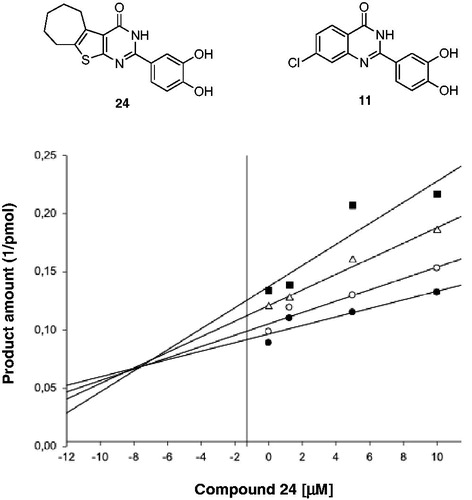
Table 1. Inhibition of wild type and p66/p51C280A mutant HIV-1 RNase H activity by compounds 2–17. 
Scheme 1. Synthetic protocol for compounds 2–13 and 15–21. Reagents and conditions: i) I2/CH3CN, rt, 6 h. ii) SnCl2/HCl 37%,−5 °C (1 h), rt (30 h).
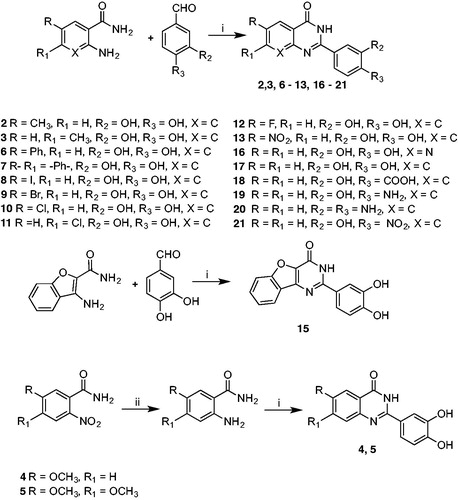
Scheme 2. Synthetic protocol for compounds 14, 22, and 23. Reagents and conditions: i) CH2Cl2/CH3CN, reflux, 40 h. ii) Pd(OAc)2/K2CO3, EtOH/H2O 3/1, rt (18 h). iii) BBr3 1 M in CH2Cl2, CH2Cl2, 0 °C (1 h), rt (3 h).
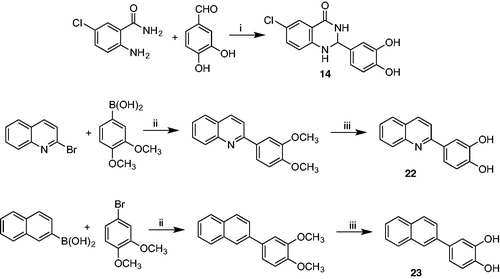
Table 2. Inhibition of HIV-1 RNase H activity by compounds 18–21. 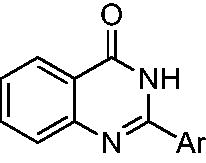
Table 3. Inhibition of wild type and p66/p51C280A mutant HIV-1 RNase H activity by compounds 22 and 23.
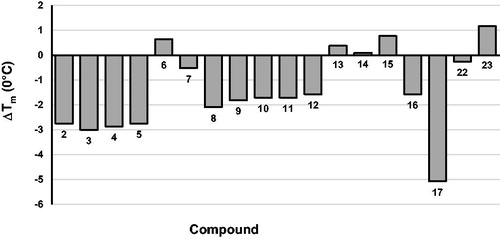
Table 4. Effect of compounds 2–17 on HIV-1 RDDP and IN functions.