Figures & data
Figure 1. Structures of the HexA inhibitors DNJNAc, NHAc-CAS, PUGNAc, NAG-thiazoline, pymetahmine, M31850, DMH-DNJNAc, LABNAc, Gal-PUGNAc and Gal-NAG-thiazoline, and of the sp2-iminosugars NOI-NJ, pFPhT-DGJ and 6S-NBI-DGJ.
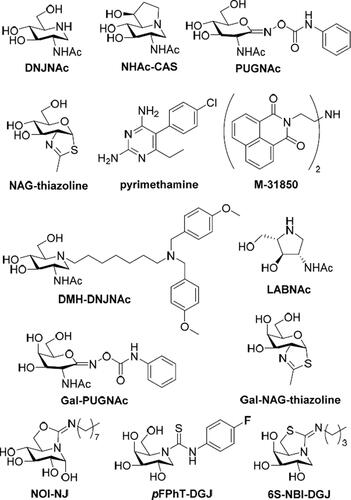
Figure 2. Schematic representation of the proposed pharmacological chaperone strategy tackling TSD causative misfolded HexA, based on active-site directed sp2-iminosugars (a generic bicyclic amphiphilic structure with a hydrophobic R substituent is depicted).
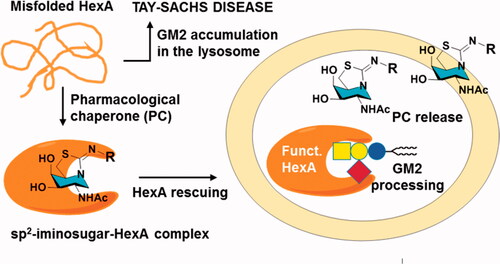
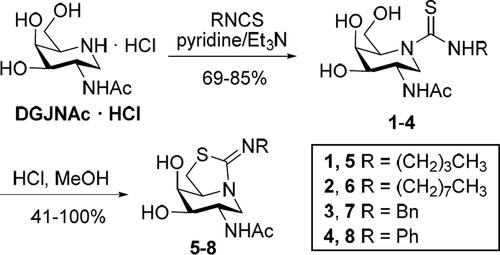
Table 1. IC50 or Ki values for sp2-iminosugars 1–8 human lysosomal hexosaminidases and recombinant human O-GlcNAcase.a
Figure 3. Predicted binding modes of phenylthiourea 4 (A; magenta sticks) and protonated octyliminothiazolidine 6 (B; orange sticks) to HexA (coordinates from PDB ID 2GK1; tan sticks) from the molecular docking experiments. Hydrogen atoms from ligands are not shown for clarity. Hydrogen bonds are represented as black dashed lines, and hydrophobic interactions as red dashed lines.
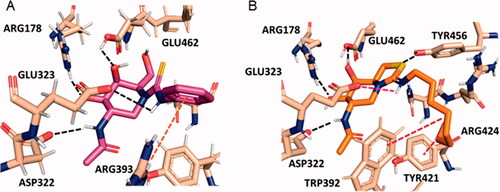
Figure 4. Effect in the HexA activity of different concentrations of the thioureas DGJNAc thioureas 1–4 and the isothiourea derivatives 5–8 in wild type (A) and G269S TSD fibroblasts (B) relative to controls in the absence of any compound. Bars represent average values ± SD (n = 3).
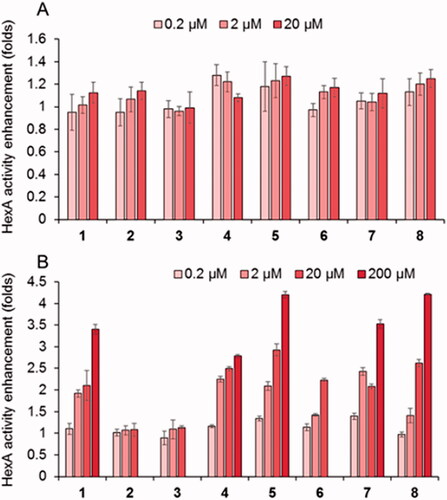
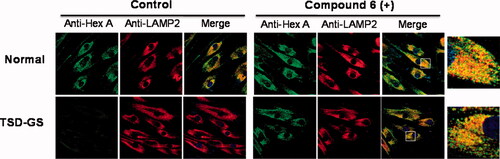
Figure 5. Effect of compound 6 on the traffic of HexA to lysosomes in WT and in TSD-GS fibroblast. Fibroblasts were treated with 20 µM complexed chaperone. Lysosomal marker (lysosome associated membrane protein 2, LAMP-2). HexA is visualised in green; in the merged images, yellow denotes colocalization in lysosomes. The shown pictures are representative of more than 100 cells in 10 randomly obtained images.