Figures & data
Figure 1. Study designs
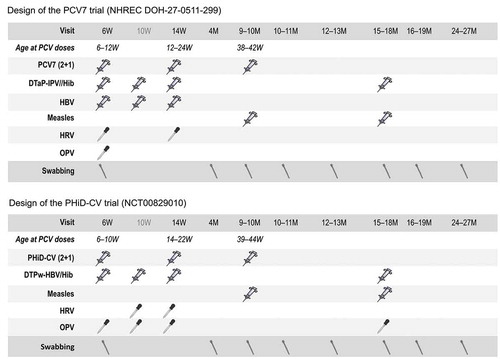
Table 1. Age at each study visit (total vaccinated cohort)
Figure 2. Colonization rates with Streptococcus pneumoniae at each visit in children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule (total vaccinated cohort)
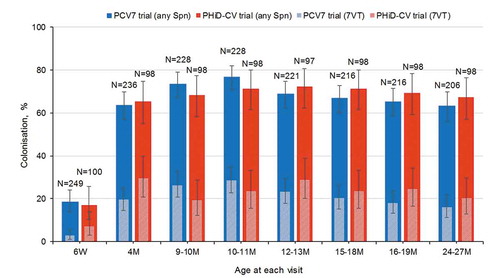
Table 2. Cumulative acquisition of Streptococcus pneumoniae serotypes (any, 7VT, non-7VT serotypes, [1, 5, 7F], 6B, 9V, 14, 19F, 23F, vaccine-related 6A and 19A serotypes) in children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule (total vaccinated cohort)
Figure 3. Kaplan-Meier estimates of the percentages of children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule and were never found colonized with Streptococcus pneumoniae (total vaccinated cohort)
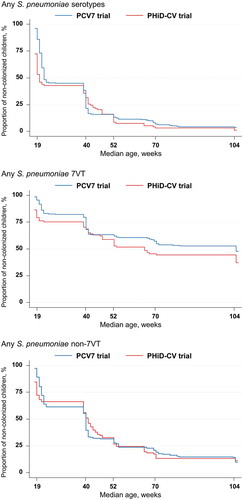
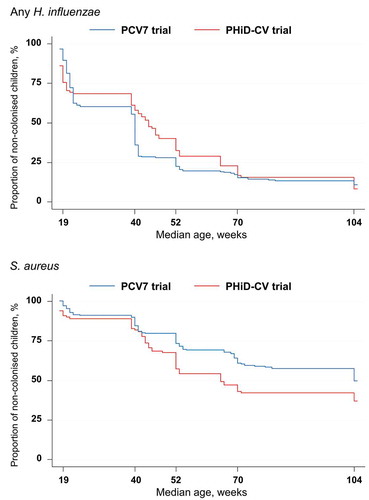
Figure 4. Kaplan-Meier estimates of the percentages children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule and were never found colonized with Haemophilus influenzae or Staphylococcus aureus (total vaccinated cohort)
Supplemental Material
Download MS Word (64 KB)Data availability statement
Anonymized individual participant data and study documents from PHiD-CV study (NCT00829010) can be requested for further research from www.clinicalstudydatarequest.com.
For the PCV7 study, data are available upon request to Wits Health Consortium and after they have signed a material transfer agreement.

