Figures & data
Figure 1. A. Overall distribution of invasive S. pneumoniae serotypes (N = 549) from 2005–2020. B. Changes in vaccine and non-vaccine serotypes in the three different age groups across the three vaccine eras of the study. PCV7 refers to isolates with serotypes 4, 6B, 9 V, 14, 18C, 19 F, and 23 F. PCV13 refers to the additional serotypes not included in PCV7: 1, 3, 5, 6A, 7 F, 19A. NVT refers to all other serotypes including those that were not typeable. Multinomial logistic regression was used to compare the percentage of VT and NVT serotypes across the 3 eras. The PCV7 era was the reference category. The bars refer to statistically significant differences in the indicated eras. C. Changes in vaccine and non-vaccine serotypes among subjects ≤5 years of age by year. PCV7 refers to isolates with serotypes 4, 6B, 9 V, 14, 18C, 19 F, and 23 F. PCV13 refers to the additional serotypes not included in PCV7: 1, 3, 5, 6A, 7 F, 19A. NVT refers to all other serotypes including those that were not typeable.
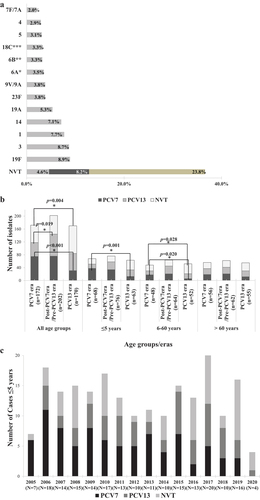
Table 1. Demographic features and clinical burden of patients with IPD from the LIPSP.
Table 2. Distribution of PCV7, PCV10, PCV13, PPV23, and other serotypes among invasive S. pneumoniae isolates recovered from all age groups during the PCV7, post-PCV7/pre-PCV13, and PCV13 eras.
Figure 2. Changes in antimicrobial resistance of S. pneumoniae over the three study eras. The percentage of penicillin, erythromycin and clindamycin non susceptible S. pneumoniae isolates are indicated in the PCV7, post-PCV7/Pre-PCV13 and PCV13 eras. Binomial logistic regression was used to compare the antimicrobial resistance within the 3 eras. The PCV7 era was the reference category. *p = 0.002, OR = 0.269, 95%CI = [0.119–0.612].
![Figure 2. Changes in antimicrobial resistance of S. pneumoniae over the three study eras. The percentage of penicillin, erythromycin and clindamycin non susceptible S. pneumoniae isolates are indicated in the PCV7, post-PCV7/Pre-PCV13 and PCV13 eras. Binomial logistic regression was used to compare the antimicrobial resistance within the 3 eras. The PCV7 era was the reference category. *p = 0.002, OR = 0.269, 95%CI = [0.119–0.612].](/cms/asset/4a513fd3-4846-4441-acb7-4f6e216f8e05/ierv_a_2143349_f0002_b.gif)
Table 3. Changes in the mortality rates in the three different age groups across the three vaccine eras of the study.
Table 4. Antimicrobial resistance profiles of major S. pneumoniae serotypes, 2005–2020.
Figure 3. Comparison of antibiotic resistance rates among different age groups over the three study eras. The percentage of Penicillin (A), Erythromycin (B) and Clindamycin (C)-non susceptible S. pneumoniae isolates are indicated in the PCV7, post-PCV7/Pre-PCV13 and PCV13 eras across three age groups. Clindamycin sensitivity was not performed for isolates from the 6-60 years age group in the PCV7 era due to technical reasons.
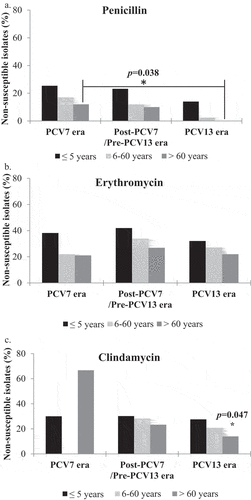
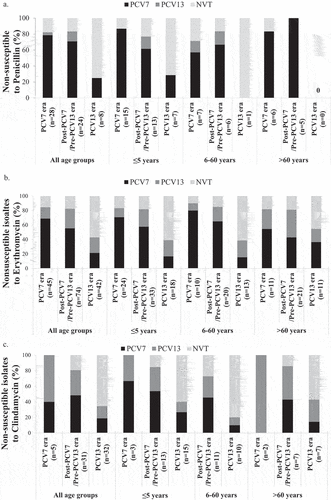
Figure 4. Impact of vaccination on antimicrobial resistance by serotypes across age groups. The contribution of vaccine and non-vaccine S. pneumoniae serotypes to penicillin (A), erythromycin (B), and clindamycin (C) resistance is indicated in the PCV7, post-PCV7/Pre-PCV13 and PCV13 eras across three age groups. Clindamycin sensitivity was not performed for isolates from the 6-60 years age group in the PCV7 era due to technical reasons. PCV7 refers to isolates with serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. PCV13 refers to the additional serotypes not included in PCV7: 1, 3, 5, 6A, 7F, 19A. NVT refers to all other serotypes including those that were not typeable.