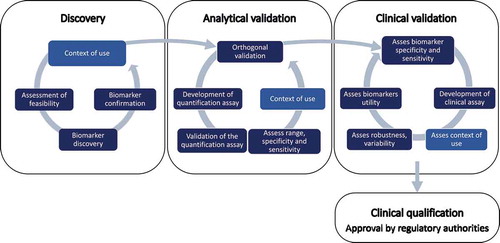
Figures & data
Table 1. List of DMD biomarkers identified. The biomarkers are ranked based on the number of publications providing evidence for association of the biomarker with DMD. Biomarkers identified in both human and animal models are listed first, followed by biomarkers identified only in DMD patients and subsequently in only DMD animal models.