Figures & data
Figure 1. Antiproliferative activity of the prodrug PR-104A (A), its DNA crosslinking metabolites PR-104H (B) and PR-104M (C), and the reference DNA crosslinking agent melphalan (D) against HCC cell lines. Cells were exposed to the drugs for 4 hr under aerobic conditions and grown for a further 5 d before staining with sulphorhodamine B. IC50 values are the mean of 3 or 4 independent experiments and error bars are SEM. Significance of pairwise differences are shown as * for P < 0.05, ** for P < 0.01 and *** for P < 0.001. One way ANOVA demonstrated significant differences between cell lines in panel B (P = 0.043) but none of the pairwise differences reached significance (P > 0.05).
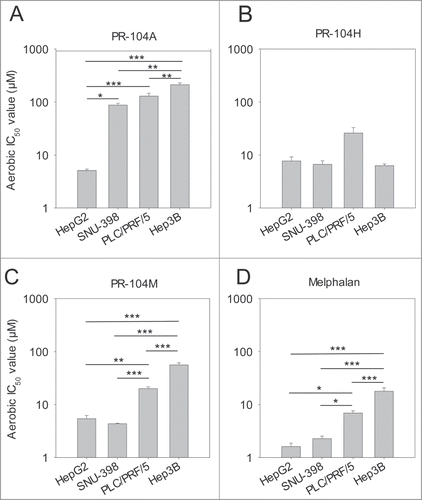
Figure 2. Clonogenic survival of HCC cell lines following a 2 hr exposure to PR-104A (A) or PR-104H (B) under aerobic or anoxic conditions. Values show the mean ± range of duplicate experiments.
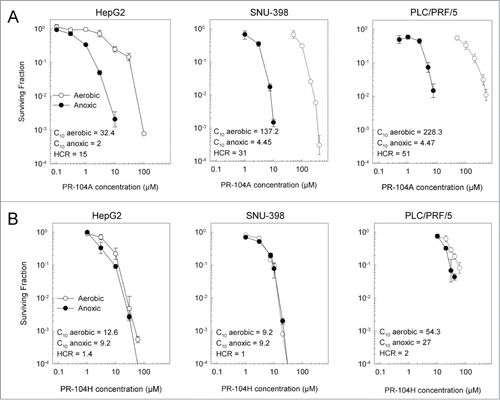
Figure 3. Metabolism of PR-104A under anoxic or aerobic conditions and expression of known PR-104A metabolizing enzymes in HCC cell lines. (A) Metabolism of PR-104A (100 μM, 1 hr) under anoxia, determined by LC-MS/MS quantitation of metabolites. Values are the mean ± SEM for 6 biological replicates in 2 independent experiments. All pairwise differences in the sum of the active metabolites (PR-104H & PR-104M) between cell lines were statistically significant (P < 0.05; ANOVA with Holm-Sidak). (B) Aerobic metabolism of PR-104A, as for A. All pairwise differences of the sum of PR-104H & PR-104M between cell lines were statistically significant except SNU-398 vs Hep3B. (C) Lysates from the same pretreatment cell populations were analyzed by Western blot to detect POR and AKR1C3. The numerical values shown are the mean ± range for the POR/ β-actin or AKR1C3/β-actin ratio for the 2 experiments. (D) Analysis of AKR1C3 enzymatic activity in aerobic HCC cells using the fluorogenic AKR1C probe coumberone (10 μM). Formation of coumberol in the presence of a specific AKR1C3 inhibitor, SN34037, was used to identify the activity attributable to AKR1C3. Values are mean and errors are SEM for 3 biological replicates. AKR1C3 activity in HepG2 was significantly higher than PLC/PRF/5 (2-tailed P = 0.0004, t-test) and was not detectable in the other lines.
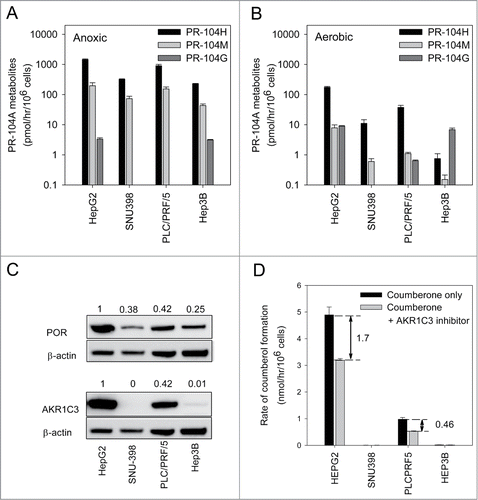
Figure 4. Levels of tumor hypoxia and AKR1C3 expression in HCC xenografts. (A) Representative images of pimonidazole binding in HCC xenograft sections. (B) Hypoxic fractions of HCC xenograft sections (mean ± SEM) calculated from imaging of pimonidazole binding (green). Only the difference between SNU-398 and PLC/PRF/5 reached statistical significance (P < 0.05). (C) Determination of AKR1C3 expression in HCC xenografts by immunohistochemistry of xenograft sections and bright-field microscopy (40x). (D) Western blot of AKR1C3, NQO1 and β-actin expression in lysates derived from in vitro cell culture or in vivo tumor xenografts. Murine liver lysate was used as a negative control. Values are the AKR1C3/actin and NQO1/actin ratios by densitometry, and errors are the range for duplicate samples.
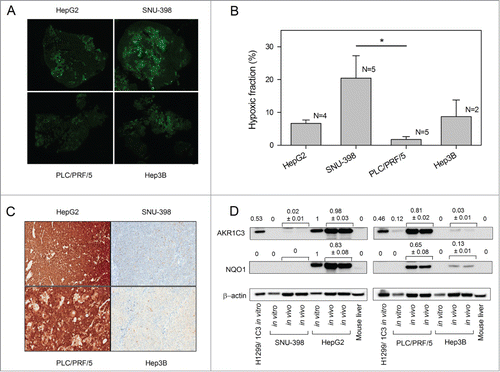
Table 1. Pathology review of AKR1C3 immunohistochemical staining in HCC xenografts and HCC surgical specimens
Figure 5. Effect of PR-104, sorafenib and the combination of both drugs on HCC tumor growth. (A) Dosing schema. (B–E) Survival curves of the time taken for tumors to reach RTV4 after treatment with vehicle, sorafenib (80 mg/kg p.o., qdx5), PR-104 (250 mg/kg i.p., qd × 6) or both drugs in combination. Significance of difference from vehicle-only controls was determined by log-rank test with Holm-Sidak multiple comparison post-test analysis.
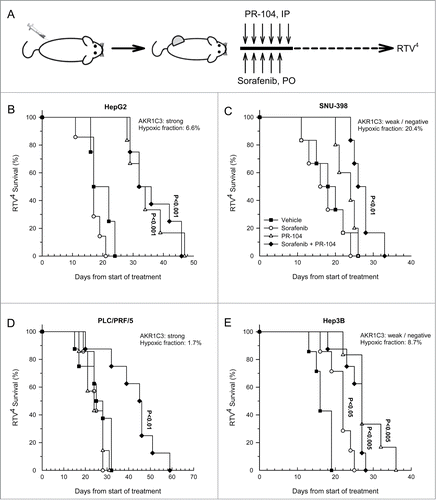
Table 2. Toxicity profile of HCC xenograft data. Body weight loss at nadir is the maximum body weight loss as a percentage of the pretreatment value (mean ± SEM). Exclusions indicate the number of animals excluded from analysis as a result of death from drug toxicity or culling due to excessive body weight loss
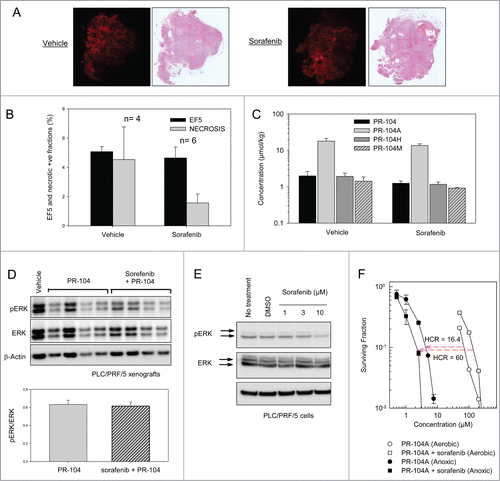
Figure 6. Effects of sorafenib on hypoxia, PR-104 metabolites and ERK phosphorylation in PLC/PRF/5 xenografts, and ERK phosphorylation and PR-104A sensitivity in PLC/PRF/5 cells in culture. (A) EF5 and H&E staining of vehicle or sorafenib-treated (80 mg/kg p.o., qdx5) PLC/PRF/5 tumors. Hypoxia probe EF5 (60 mg/kg, i.p.) was administered 24 hr after the final sorafenib dose, and tumors recovered 4.5 hr later. Representative EF5 and H&E images are shown. (B) Quantitation of area of hypoxia and necrosis from tumors treated with vehicle or sorafenib as in A. (C) Concentrations of PR-104 and its metabolites in tumors 30 min after dosing with PR-104 (250 mg/kg, IV), following pre-treatment with vehicle or sorafenib for 5 d as in A. (D) Western blot of pERK, ERK and β-actin from lysates of tumors from vehicle, PR-104 or PR-104+sorafenib-treated mice, using the same doses and schedules as in . Tumors were excised 30 min after the final PR-104 dose. Each lane is for a single tumor. Ratios of pERK/ERK (N = 4) are shown below the Westerns. (E) Western blot of pERK, ERK and β-actin from lysates of PLC/PRF/5 cells following a 2 h treatment with a range of sorafenib concentrations in vitro. (D) Clonogenic survival of PLC/PRF/5 cells under aerobic or anoxic conditions following a 2 hr exposure to PR-104A either in media only (circles) or following 3 day incubation with 8 μM sorafenib (squares).