Figures & data
Figure 1. The structure, stability and bispecificity of Bi-Ab. Structure of the anti-EGFR/VEGFR2 bispecific antibody (A) VLE, variable light region of cetuximab; VHE, variable heavy region of cetuximab; VLK, variable light region of mAb-04; VHK, variable heavy region of mAb-04. SDS-PAGE analysis of the purified Bi-Ab (B) Lane M, marker; lane 1, cetuximab; lane 2, mAb-04; lane 3, Bi-Ab. SDS-PAGE was used to analyze the thermostability of Bi-Ab (C) Lane M, marker; lane 1–6, sample incubated for 0, 3, 6, 9, 12 and 15 d at 37℃. The surface plasmon resonance spectroscopy analysis (D) VEGFR2 was injected after Bi-Ab were flowed over the EGFR-immobilized sensor chip. RU, resonance units.
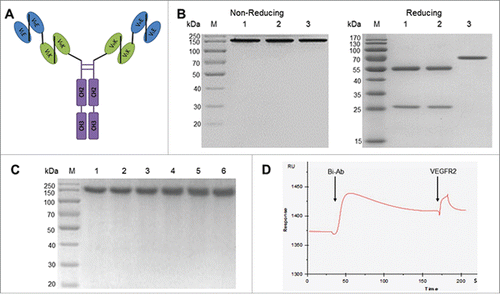
Table 1 SPR analysis of the binding affinities of antibodies to EGFR or VEGFR2.
Figure 2. The binding of Bi-Ab to recombinant EGFR/VEGFR2 ectodomains and membrane-associated EGFR/VEGFR2. Surface plasmon resonance spectroscopy was used to analysis the binding kinetics of Bi-Ab to recombinant EGFR and VEGFR2 ectodomains ((A) and B). The equilibrium dissociation rate constant (KD) of Bi-Ab to EGFR or VEGFR2 were 1.45×10 −9M and 2.37×10 −9M respectively. Flow cytometry was used to investigate the binding of Bi-Ab to membrane-associated EGFR and VEGFR2 ((C)- F). The histogram overlay showed that Bi-Ab binds to HT-29 and SKOV-3 cells line with a relatively high binding levels.
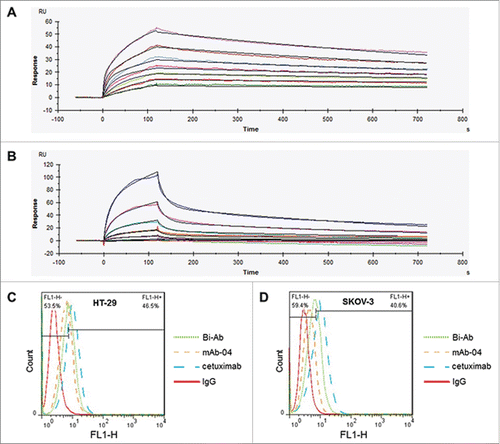
Figure 3. Bi-Ab inhibits phosphorylation of EGFR and VEGFR2 and down-regulates AKT and MAPK signaling. (A) Western blot to show the inhibition on phosphorylation of EGFR, VEGFR2, AKT and Erk1/2 in HT-29 and SKOV-3 cells after antibodies treatment. ((B) and C) Phosphorylation level of AKT and Erk1/2 in HT-29 and SKOV-3 cells. The data presented as the mean ± SD, are from a representative experiment, n = 3. *P < 0.05; **P < 0.01.
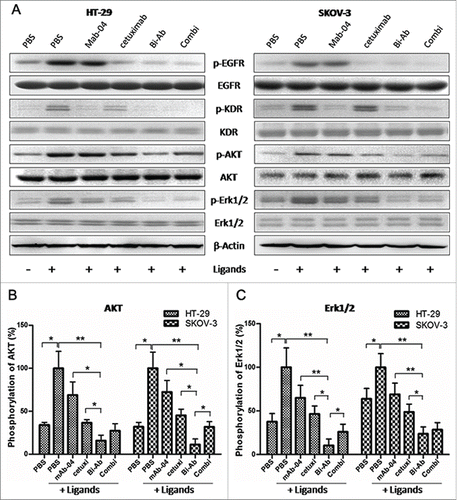
Figure 4. Bi-Ab showed the most effective inhibition of proliferation on HT-29 and SKOV-3 cells compared to mAb-04, cetuximab or Combi with EGF and VEGF stimulated ((A)and B). Three independent experiments were performed in triplicate, the means ± SD of triplicate experiment are shown, *P < 0.05; **P < 0.01 versus treatment with Bi-Ab treatment. Photomicrographs of transwell invasion assay indicated that Bi-Ab could effectively inhibit the invasion of HT-29 and SKOV-3 cells induced by EGF and VEGF ((C)and D). Quantitative analysis of the transwell invasion assay showing that Bi-Ab treatment significantly increased the inhibition of HT-29 and SKOV-3 cells invasion when compared to mAb-04 and cetuximab. The data presented as the mean ± SD, are from a representative experiment, 5 independent experiments were performed in triplicate, *P < 0.05; **P < 0.01. Percent ADCC of the antibodies on HT-29 and SKOV-3 (E). The data presented as the mean ± SD, each antibody was tested in triplicate, the assays were repeated once, n = 3, *P < 0.05.
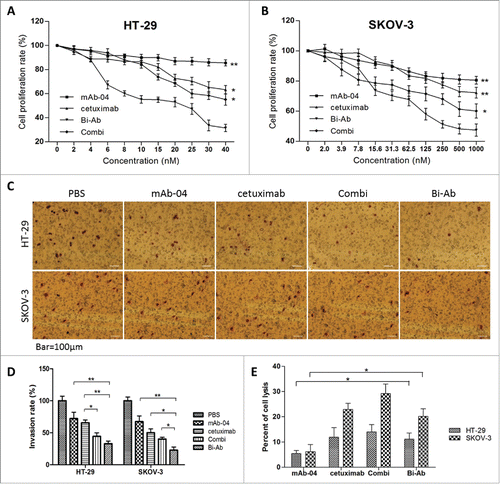
Figure 5. The apoptosis was analyzed by flow cytometry, endothelial tube formation was performed using HUVECs tube formation assay. ((A)and C) Bi-Ab treatment increased apoptosis in SKOV-3 cells than cetuximab and mAb-04 alone or Combi, but not HT-29. (B) HUVEC tube-like photomicrographs showing the significant effects of Bi-Ab on HUVECs tube formation. (D) Similar to the Combi, Bi-Ab demonstrated relatively more potent restraining effect on tube formation by HUVEC cells compared to mAb-04 or cetuximab. Three independent experiments were performed in triplicate, the means ± SD of triplicate experiment are shown (*P <0 .05; **P <0 .01 vs. Bi-Ab treatment).
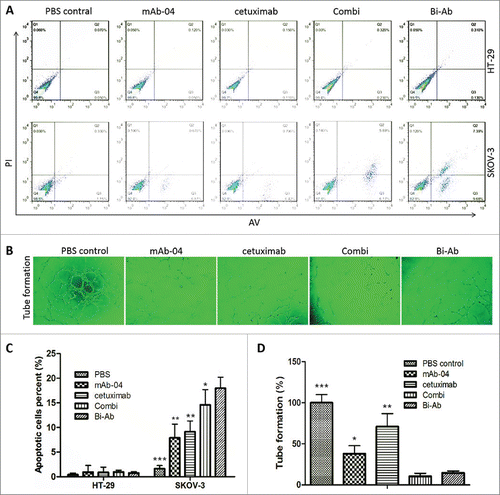
Table 2 Median survival and 100-day survival rate (%).
Figure 6. The Bi-Ab shows potent antitumor effect on HT-29 and SKOV-3 tumor xenografts in nude mice. ((A) and B) Bi-Ab suppressed tumor growth, tumor diameter was measured with a vernier caliper (*P < 0.05; **P < 0.01; ***P <0 .005 versus treatment with Bi-Ab). The survival rates of HT-29 and SKOV-3 tumor-bearing mice ((C)and D). The median survival and terminal survival rate were shown in .
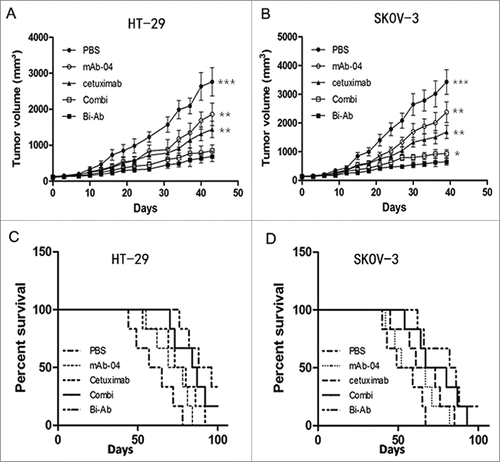
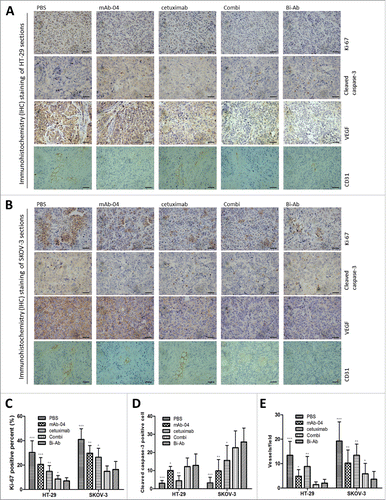
Figure 7. Immunohistochemical analysis was used to measure the effect of Bi-Ab treatment on proliferation, apoptosis and angiogenesis in vivo. In HT-29 (A) and SKOV-3 (B) tumors, proliferation and apoptosis were evaluated with Ki-67 and cleaved caspase-3 staining methods respectively, VEGF and CD31 were used to evaluate tumor angiogenesis. (C) Proliferative cells (Ki-67 positive cells) decreased in Bi-Ab-treated HT-29 and SKOV-3 tumors, and it was more effective compared to mAb-04- and cetuximab-treated tumors. (D) Apoptotic cells (cleaved caspase-3 positive cells) increased in Bi-Ab-treated HT-29 and SKOV-3 tumors. (E) The density of CD31-positive blood vessels decreased in Bi-Ab-treated HT-29 and SKOV-3 tumors. The data presented as the mean ± SD, are from a representative experiment, n = 5, *P < 0.05; **P < 0.005; ***P < 0.0005 vs. Bi-Ab treatment.