Figures & data
Figure 1. Schematic representation illustrating the components and interactions within the tumor microenvironment.
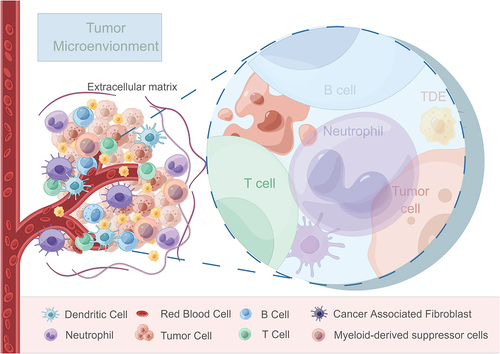
Figure 2. Schematic representation depicting the process of exosome biogenesis and release. A. The steps involved in exosome biogenesis: ① Invagination of the plasma membrane. ② Formation of early endosomes. ③ Maturation of early endosomes into late endosomes, which develop into multivesicular bodies (MVBs). The membrane invagination of MVBs results in the formation of intraluminal vesicles (ILVs), which then fuse with the plasma membrane and release ILVs as exosomes. B. Schematic representation illustrating the structure of exosomes.
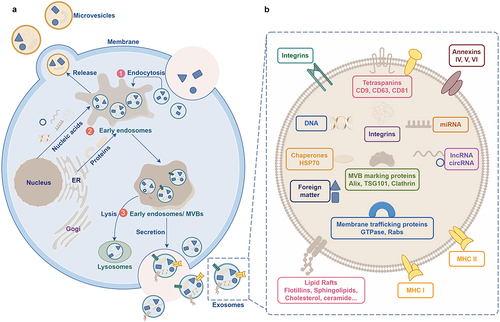
Figure 3. Schematic diagram illustrating key steps in the biogenesis and transport of tumor-derived exosomes in a hypoxic tumor microenvironment. ① Hypoxia influences cargo synthesis and sorting within exosomes. ② Hypoxia affects cargo loading into exosomes. ③ Hypoxia regulates exosome biogenesis by influencing the degradation of transporters (such as TSG101). ④ the hypoxic environment regulates exosome release by influencing the degradation of Rab GTPases (such as Rab27a). ⑤ the hypoxic environment regulates exosome release by affecting SNARE complexes specifically required for multivesicular body (MVB) fusion with the plasma membrane. ⑥ the hypoxic microenvironment influences the transport of tumor-derived exosomes to recipient cells. ⑦ the hypoxic microenvironment affects the recognition and uptake of tumor-derived exosomes by recipient cells.
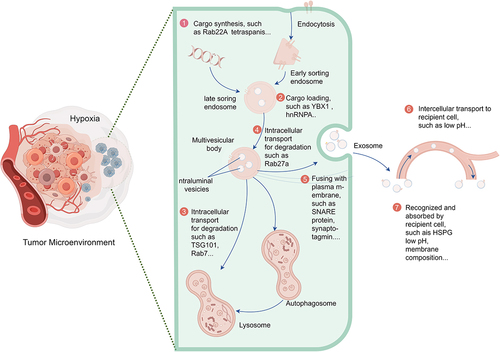
Figure 4. Regulation of immune cells in an immunosuppressive microenvironment by tumor-derived small extracellular vesicles (TDSEVs). ① TDSEVs activate immune cells and promote antitumor immunity by carrying tumor-associated antigens (TAA) in their cargo. ② TDSEVs play a crucial role in the conversion of M0 macrophages to M2 macrophages, commonly referred to as tumor-associated macrophages (TAMs). ③ TDSEVs enhance the inhibitory effect of myeloid-derived suppressor cells (MDSCs). ④ it has been observed that TDSEVs can inhibit the function of immune cells.
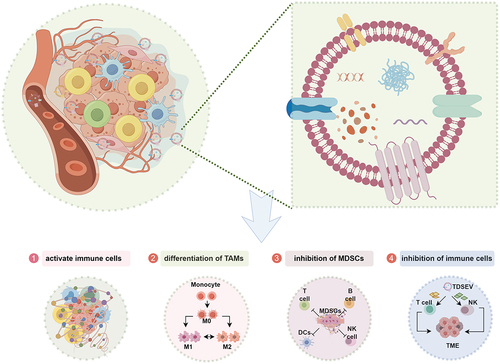
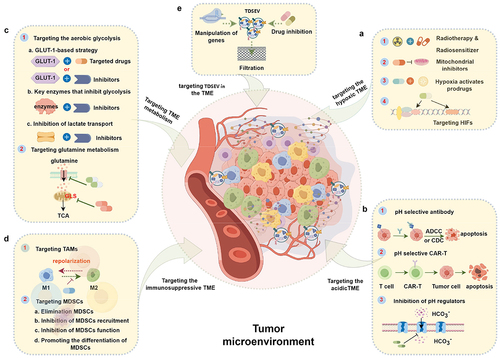
Figure 5. Therapeutic strategies based on targeting characteristics of the tumor microenvironment (TME) and tumor-derived small extracellular vesicles (TDSEVs). A. Targeting the hypoxic TME. These strategies mainly include the use of electron affinity radiosensitizers, enhancing tumor tissue oxygenation, hypoxia-activating prodrugs (HAPs), and targeting hypoxia-inducible factors (HIFs) and downstream targets. B. Targeting the acidic TME. This involves the development of acidic pH-selective antibodies, acidic pH-selective chimeric antigen receptor T (CAR-T) cells, and targeted inhibition of pH modulators. C. Targeting TME metabolism. Major strategies focus on aerobic glycolysis and glutamine metabolism pathways. D. Targeting the immunosuppressive TME. Strategies involve focusing on tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). E. Targeting TDSEVs in the TME. Major strategies include genetic manipulation, drug inhibition, and clearance of TDSEVs.