Figures & data
Figure 1 (See previous page). Neural differentiation of mESC. (A–C) Scheme, phase contrast microscopy pictures, and immunocytochemistry of successive phases and cellular stages during neural differentiation of mESC. (A) Undifferentiated mESC formed colonies. After neural differentiation induction, pluripotent mESC developed into neuroepithelial precursor cells (NPCs). By day 5, these NPCs were organized in rosette-formations, giving rise to developing neurons around days 8 to 12 (neurogenesis) and to astrocytes by day 15 (gliogenesis). Processes extended from the cell clones by day 8, sprouted further and formed networks around day 12, resulting in a compact network of neuronal and glial fibers by day 19. Cells in the center of rosettes still proliferated, thereby establishing large cell clusters. Red dots depict centrosomes. (B) Phase contrast microscopy images illustrating morphological changes of mESC during neural differentiation. Scale bars 20 μm. (C) Cdk5rap2 (red) adopted a strongly polarized position in the center of rosettes from day 5 to day 12; this formation slowly disappeared around day 15. DNA was stained with DAPI (blue). Immunofluorescence images, scale bars 20 μm. (D) mESC were immunopositive for the stem cell marker Oct4 (red) on days 0 and 5, but not on day 8 after neural differentiation induction. DNA was stained with DAPI (blue). Immunofluorescence images, scale bars 10 μm. (E) On day 0 and 5 about 97% and 91%, respectively, of all cell groups were Oct4-immunopositive (n = 3 per group, one-way ANOVA, P < 0.0001, Bonferroni's Multiple Comparison Test), while at day 8 nearly all of them (98%) were immunonegative. (F) Presence of cells positive for Cdk5rap2, stem cell markers (Oct4, Lin28) and markers for differentiated cells (Map2, NeuN, Gfap) during neural differentiation analyzed by immunocytochemistry: -, not present; +/−, first positive cells detectable; +, some positive cells; +++, many positive cells. (G) Cdk5rap2 mRNA levels, analyzed by qPCR, in undifferentiated mESC and during neural differentiation in vitro (n=6 per group, one-way ANOVA, P < 0.0001, Bonferroni's Multiple Comparison Test). Cdk5rap2 expression decreased at the beginning of differentiation and rose again, especially at the later stage (day 19) when astrocytes appeared in the differentiating mESC culture. *P < 0.05, **P < 0.01, ***P < 0.001.
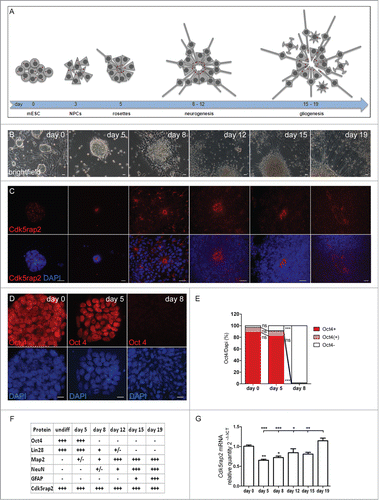
Figure 2. Cdk5rap2 in neurons and glial cells. Cdk5rap2 (red) was present in postmitotic cells immunopositive for (A) Map2 (green; marker for early neurons) and (C) Gfap (green; marker for glial cells), but not in (B) NeuN-positive cells (green; marker for mature neurons). Arrowheads, Cdk5rap2-positive centrosomes; arrows, NeuN-positive and Cdk5rap2–negative cells; confocal images, scale bars 10 μm.
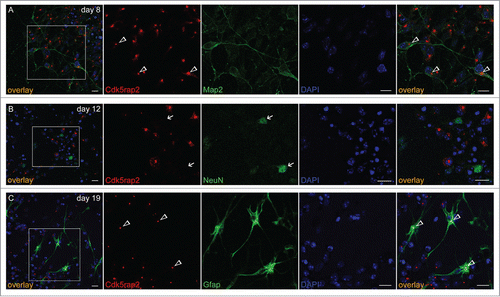
Figure 3. Proliferation defect of undifferentiated mESC with Cdk5rap2 downregulation, but no enhanced spontaneous apoptosis. (A) Phase contrast microscopy pictures of cultures at DIV3; note the reduced density and size of cell clusters particularly for clones sh1 and sh2. Scale bar 100 μm. (B) Cell viability of undifferentiated mESC days in vitro (DIV) 1–3 of culturing (n = 4 −12 per group, one-way ANOVA, P < 0.0001, Bonferroni's Multiple Comparison Test) demonstrated a reduced growth of Cdk5rap2 shRNAi cell cultures. (C) Proliferation and (D) relative apoptosis of undifferentiated mESC at DIV3 (n = 4 −6 per group, one-way ANOVA, P < 0.0001, Bonferroni's Multiple Comparison Test). Proliferation was strongly or moderately reduced for strongly (sh1 and sh2) or intermediately Cdk5rap2-downregulated clones (sh3 and sh4), respectively. Apoptosis varied between shRNAi clones and was not increased compared to control cells at DIV3. Abbreviations: Co, control; scr, scramble; sh1–4, shRNAi clones 1–4; ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
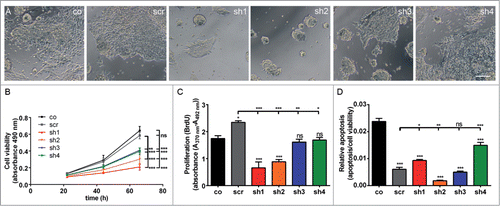
Figure 4. Loss of mESC cell culture during neural stem cell differentiation through strong Cdk5rap2 downregulation. (A) Low magnification of mESC culture growth after neural differentiation induction depicted complete loss of clone sh2 (similar results for sh1, data not shown) and only few surviving cell groups in clone sh4 (similar results for sh3, data not shown). Pictures of day 15 and 19 are similar to day 12; results for scr are similar to co (data not shown). Phase contrast microscopy pictures, black scale bar 1 mm. Inset pictures show representative cell clusters in higher magnification. White scale bars 50 μm. (B) Cell cluster number and (C) size at day 5 and 8 of neural differentiation (n = 6 per group, one-way ANOVA, P < 0.0001, Bonferroni's Multiple Comparison Test). At day 5, cell cluster number and size was strongly reduced in clones with strongly downregulated Cdk5rap2 (sh1, sh2). Cell clusters of clones with intermediately downregulated Cdk5rap2 (sh3, sh4) were reduced in number, but not in size. At day 8, cultures of clones sh1 and sh2 were completely lost. In cultures of clones sh3 and sh4 few cell clusters with normal size were present. Abbreviations: Co, control; scr, scramble; sh1–4, shRNAi clones 1–4; ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
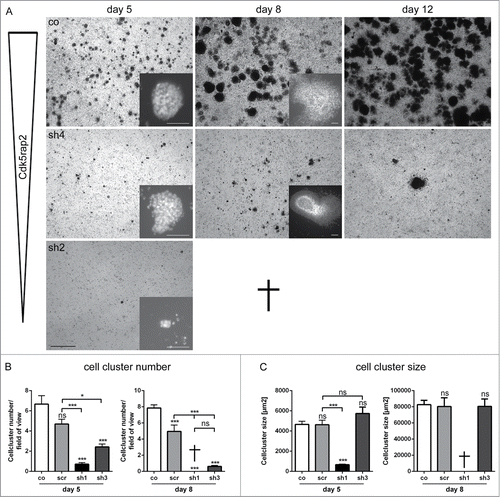
Figure 5. Severely reduced proliferation and apoptosis of neuroepithelial cells in Cdk5rap2-depleted mESC. Representative, sequential bright field live-cell imaging pictures starting at day 3 after differentiation induction (imaging 65h, pictures taken every 7 min, scale bar 50 μm). (A) In control cultures, cells proliferated rapidly, leading to an extension of the cell clusters, and subsequently migrated into the periphery while still keeping contact to adjacent cells. Monolayers between cell clusters were formed. (B) Cdk5rap2-depleted cell clusters were already much smaller at the beginning of live-cell imaging and cells at the edge of these clusters lost contact to adjacent cells and underwent cell death. Thereby, no viable cells were left at day 5 after differentiation induction (images 50 and 65 h), and no migrating cells were visible. Abbreviations: Co, control; scr, scramble; sh1–4, shRNAi clones 1–4.
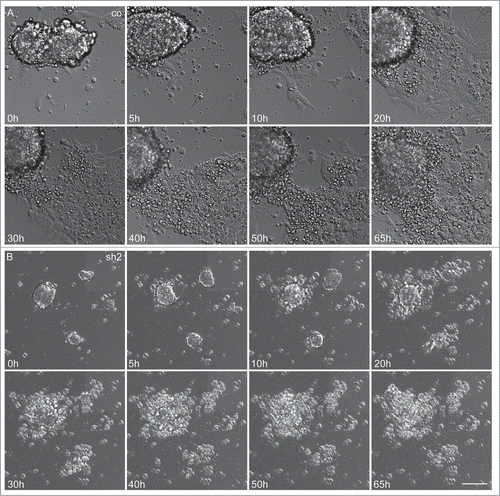
Figure 6 (See previous page). Accumulating proliferation defect and apoptosis of neurally differentiating mESC. (A) Cell viability and (B) relative apoptosis of neurally differentiating mESC. Severely affected cell viability in clones with strongly downregulated Cdk5rap2 (sh1, sh2) as well as in clone sh3, becoming clearly apparent at day 5 after neural differentiation induction. Significant increase of apoptosis in clone sh1 on days 2 and 3 after differentiation induction. (C) Representative Immunofluorescence pictures at day 8 depicting the typical loss of Oct4-positivity (red) in control and scramble cell groups, while Cdk5rap2-downregulated clones still contained Oct4-positive cells in their center; DNA was stained with DAPI (blue). Scale bar 100 μm. (D) Oct4 quantification of undifferentiated (day 0) mESC and at day 5 and 8 of neural differentiation (n = 3 per group, 2-way ANOVA, Bonferroni's Multiple Comparison Test). No significant differences in Oct4 staining were detected between the undifferentiated clones. At day 5 after differentiation induction, in clone sh3 significantly less cell groups were Oct4-positive (***P < 0.001), but significantly more cell groups with Oct4-positive cells in the center (**P < 0.01) were present compared to the control mESC. At day 8, in clone sh3 significantly less cell groups were Oct4-negative (***P < 0.001) compared to control/scramble mESC and significantly more were Oct4-positive (*P < 0.05) or had Oct4-positive cells in their center (***P < 0.001). No premature loss of Oct4-positivity was detected in Cdk5rap2-downregulated mESC. (E) In control and scramble cultures, NeuN-positive Neurons (green) were distributed harmoniously in the periphery of rosette-formations on day 15, while in downregulated clones they arranged in abnormal clusters (clone sh3, similar results for sh4, data not shown); Cdk5rap2 (red), DAPI (blue); confocal pictures, scale bar 50 μm. (F) Quantification of NeuN-positive cells relative to DAPI at day 15 after neural differentiation induction revealed an increase of NeuN-positive mature neurons in clone sh3. (G) No premature spontaneous differentiation of Cdk5rap2-depleted mESC. At day 6 after withdrawal of mLIF, cells in sh2 were still Oct4-positive (red), while most cells in the control were already Oct4-negative at day 5; DNA was stained with DAPI (blue). Immunofluorescence pictures, scale bars 50 μm. (H) Quantification of Oct4-positive cells during spontaneous differentiation after withdrawal of mLIF and amount of cells determined indirectly by measurement of DAPI-positive area per view field. At day 4 after withdrawal of mLIF no significant differences could be found between the control, scramble and shRNAi clones sh2 and sh3. At day5 and 6 the relative amount of Oct4-positive cells was significantly increased in the Cdk5rap2 strongly downregulated clone sh2, whereas the number of all DAPI-positive cells was dramatically reduced. Results for the clone with intermediate Cdk5rap2 downregulation (sh3) showed the same trend, but were not significant.
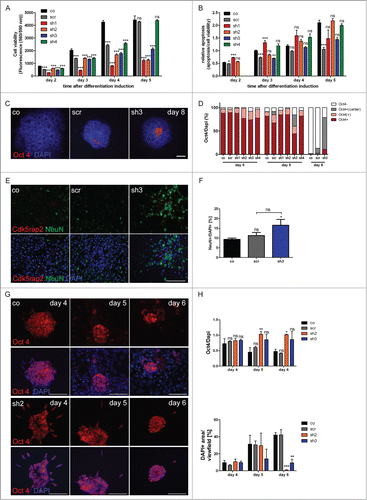
Figure 7. Loss of Cdk5rap2 does not affect non-neural mESC differentiation into beating cardiomyocytes. (A) Scheme of assay to evaluate mESC differentiation into beating cardiomyocytes according to the validated embryonic stem cell test.Citation24 (B) Normal cardiac differentiation was obtained for a Cdk5rap2 shRNAi mESC clone with strong Cdk5rap2 downregulation (sh1) as well as for control and scramble (n = 3 experiments, 24 wells each, one-way ANOVA, p = 0.1238, Bonferroni's Multiple Comparison Test, ns, not significant). Abbreviations: Co, control; scr, scramble; sh1–4, shRNAi clones 1–4; ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
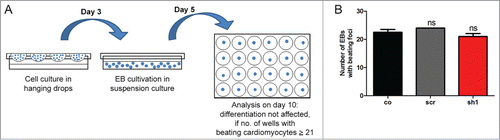
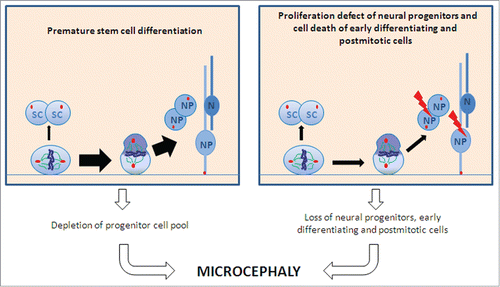
Figure 8. Schematic of proposed pathomechanisms underlying microcephaly in MCPH. A premature shift from symmetric to asymmetric cell division with a subsequent depletion of the progenitor pool (left part) and proliferation defects of differentiating stem cells and cell death of proliferative cells and early postmitotic cells (right part) lead to microcephaly. Abbreviations: SC, stem cell; N, neuron; NP, neural precursor.