Figures & data
Figure 1. Hierarchical DSB patterning. Regulation of DSB position during prophase I is achieved by means of a hierarchical collection of processes operating via three major nodes: hotspot designation, proactive regulation, and reactive regulation. Rather than acting in isolation, these processes interconnect — sculpting the final DSB distribution with a high degree of complexity (see text for further details). Further to those spatial mechanisms outlined in this review, meiotic recombination is additionally subject to temporal regulation (reviewed in Ref. 2 and Ref. 9), ensuring, for example, that DSB formation occurs post-replication and that the process is ultimately shutdown by homologous chromosome synapsis. Differences in the usage and timing of replication origin activity, and in the relative efficiency of homolog engagement, may therefore permit such processes to contribute, in a generalized manner, to spatial regulation.
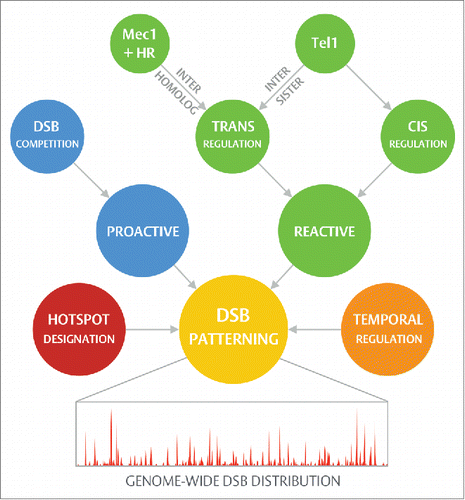
Figure 2. Meiotic hotspot designation. Left - Gatekeeper factors - predictors of recombination. Hotspot designation differs significantly between species. While lower eukaryotes (S. pombe and S. cerevisiae) rely upon a set of passive, low-impact factors, higher eukaryotes (H. sapiens and M. musculus) utilize the multi-functional histone-trimethyltransferase, PRDM9, to guide recombination through the binding of PRDM9 consensus sequences [see text for further details]. Outside of these well-characterized systems, several further organisms display a number of unique properties. Within the canine lineage (C. familiaris), PRDM9 is unexpectedly non-functional—having inactivated between ∼7-9Mya—with GC-richness instead serving as a robust predictor of Spo11-activity.Citation77–79 In contrast to the majority of model organisms, insects (D. melanogaster) and worms (C. elegans) appear devoid of traditional hotspots—consistent with the co-localization of short, repeating sequences with sites of recombination.Citation80–82 A role for non-PRDM9 sequence motifs within recombination, however, does not preclude the existence of hotspots, as noted within A. thaliana and S. pombe.Citation7,83-86 Right - Layers of hotspot designation within S. cerevisiae. Canonical hotspot designation, as seen within S. cerevisiae, requires the co-occurrence of several factors in a specific fashion in order to unlock the potential for a region to initiate recombination [see text for further details].
![Figure 2. Meiotic hotspot designation. Left - Gatekeeper factors - predictors of recombination. Hotspot designation differs significantly between species. While lower eukaryotes (S. pombe and S. cerevisiae) rely upon a set of passive, low-impact factors, higher eukaryotes (H. sapiens and M. musculus) utilize the multi-functional histone-trimethyltransferase, PRDM9, to guide recombination through the binding of PRDM9 consensus sequences [see text for further details]. Outside of these well-characterized systems, several further organisms display a number of unique properties. Within the canine lineage (C. familiaris), PRDM9 is unexpectedly non-functional—having inactivated between ∼7-9Mya—with GC-richness instead serving as a robust predictor of Spo11-activity.Citation77–79 In contrast to the majority of model organisms, insects (D. melanogaster) and worms (C. elegans) appear devoid of traditional hotspots—consistent with the co-localization of short, repeating sequences with sites of recombination.Citation80–82 A role for non-PRDM9 sequence motifs within recombination, however, does not preclude the existence of hotspots, as noted within A. thaliana and S. pombe.Citation7,83-86 Right - Layers of hotspot designation within S. cerevisiae. Canonical hotspot designation, as seen within S. cerevisiae, requires the co-occurrence of several factors in a specific fashion in order to unlock the potential for a region to initiate recombination [see text for further details].](/cms/asset/4c507b3f-2f6c-4fd6-9eb5-2ec3378b9c81/kccy_a_1093709_f0002_c.gif)
Figure 3. DSB interference within S. cerevisiae. Top - Meiotic chromosomes organize into linear arrays of chromatin loops bound by a proteinaceous axis. Prior to DSB formation, we propose that a sub-population of chromatin loops in any given cell exist in a pre-activated state, “priming” hotspots for usage. An attractive candidate for pre-activation may be the tethering of loop sequences to the chromosome axis as proposed within the tethered loop-axis model.Citation25–29 While pre-activation may be an unavoidable byproduct of the way in which DSB formation is setup, it may exist to actively underscore hotspot selection—the process by which the cell determines which of the available hotspots to utilize in any given round of meiosis. Middle - Within wild-type cells, a DSB at any given primed hotspot triggers a Tel1ATM- and distance-dependent suppressive effect (DSB interference), repressing DSB formation at adjacent intra-loop hotspots and within neighboring regions across ∼70-100kb in a reactive, DSB-dependent manner.Citation46 Bottom — In the absence of Tel1, DSB interference is abrogated, enabling adjacent DSBs to arise independently over mid-long range distances (>20-100kb). Over short distances (<20kb), loss of Tel1 activity unmasks the effects of pre-activation within singular loop-domains—manifesting as patches of “negative interference” (as calculated by the standard interference formula: 1-OBS/EXP) due to the concerted formation of adjacent DSBs at frequencies greater than expected from the population average [see text for further details].
![Figure 3. DSB interference within S. cerevisiae. Top - Meiotic chromosomes organize into linear arrays of chromatin loops bound by a proteinaceous axis. Prior to DSB formation, we propose that a sub-population of chromatin loops in any given cell exist in a pre-activated state, “priming” hotspots for usage. An attractive candidate for pre-activation may be the tethering of loop sequences to the chromosome axis as proposed within the tethered loop-axis model.Citation25–29 While pre-activation may be an unavoidable byproduct of the way in which DSB formation is setup, it may exist to actively underscore hotspot selection—the process by which the cell determines which of the available hotspots to utilize in any given round of meiosis. Middle - Within wild-type cells, a DSB at any given primed hotspot triggers a Tel1ATM- and distance-dependent suppressive effect (DSB interference), repressing DSB formation at adjacent intra-loop hotspots and within neighboring regions across ∼70-100kb in a reactive, DSB-dependent manner.Citation46 Bottom — In the absence of Tel1, DSB interference is abrogated, enabling adjacent DSBs to arise independently over mid-long range distances (>20-100kb). Over short distances (<20kb), loss of Tel1 activity unmasks the effects of pre-activation within singular loop-domains—manifesting as patches of “negative interference” (as calculated by the standard interference formula: 1-OBS/EXP) due to the concerted formation of adjacent DSBs at frequencies greater than expected from the population average [see text for further details].](/cms/asset/c889f877-4104-4bb4-88e3-34726ac2ebb8/kccy_a_1093709_f0003_c.gif)
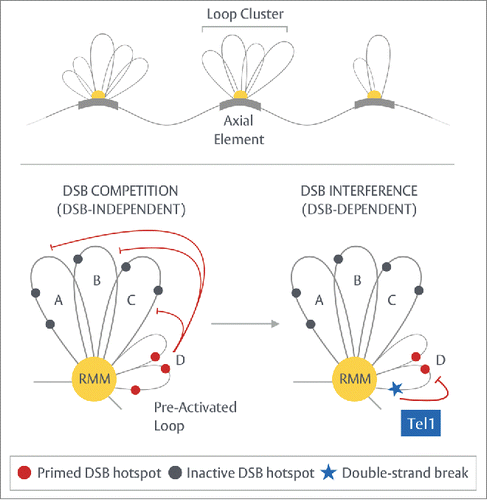
Figure 4. Prospective “loop cluster” model of DSB competition. Top - During early prophase I, short stretches of axial element nucleate at scattered regions across each chromosome.Citation73,74 Upon this platform, the first meiotic loops may begin to assemble, associating together into individually acting, isolated units. Bottom – Building upon the tethered-loop axis model, we propose that within any such clustered unit, limited availability of, or access to, essential factors such as RMM (the Rec114-Mei4-Mer2 complex), coupled to a differential ability of each loop to establish a tether, could generate DSB competition by means of competitive tethering—lowering the frequency of DSB formation within the remainder of the associated loops in a proactive, DSB-independent manner. Under wild-type conditions, DSB formation subsequently induces Tel1ATM-dependent DSB interference—a process that may inhibit or dismantle cluster units thereby suppressing further DSB formation in the immediate region. As illustrated here, the apparent Tel1-independency of DSB competition suggests the strong, repressive effect observed around strong hotspots is in fact, a composite of two distinct processes.