Figures & data
Figure 1. Periodic expression of Kv10.1 along the cell cycle. A. HeLa cells were synchronized with a double thymidine block and released into fresh medium. Total cell lysates were prepared at the indicated time points and endogenous Kv10.1 was precipitated. Analysis by SDS-PAGE and Western blotting using anti-Kv10.1, anti-cyclin A2 and anti-Cyclin B1 showed that Kv10.1 expression changes along the cell cycle, with a peak expression between 8 and 12 h corresponding to G2/M. Calnexin and Actin were used as loading controls. B. Asynchronous HeLa cells were labeled with anti-Cyclin B1, anti- p-Histone H3 (Ser 28) and anti-Kv10.1. Cells in G2, as evidenced by cytoplasmic Cyclin B1 signal, as well as mitotic cells (nuclear Cyclin B1 and p-H3 Ser 28) showed Kv10.1 reactivity at the plasma membrane. C. Cyclin B1-positive cells were localized to the proliferative compartment, at the bottom and sides of the crypt. Kv10.1 positive cells were also found in the proliferative compartment of the colon crypt. White arrows indicate Kv10.1 and Cyclin B1 expressing cells. Scale bar 20 µm.
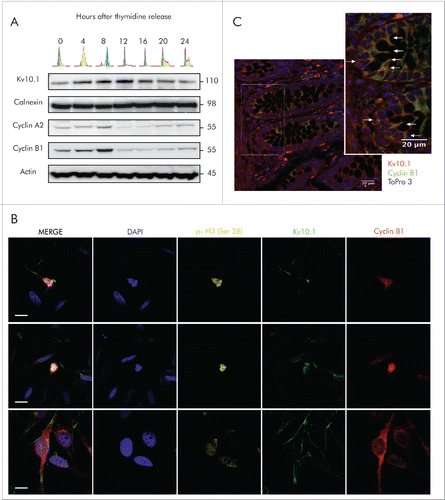
Figure 2. Kv10.1 can be detected at the cytoplasmic bridge in cells undergoing cytokinesis, and it is also present on the cell surface of cells at G1 phase, as determined by Cdt1 expression. HeLa cells were labeled with anti- Cdt1 and anti-Kv10.1. Scale bar 20 µm.
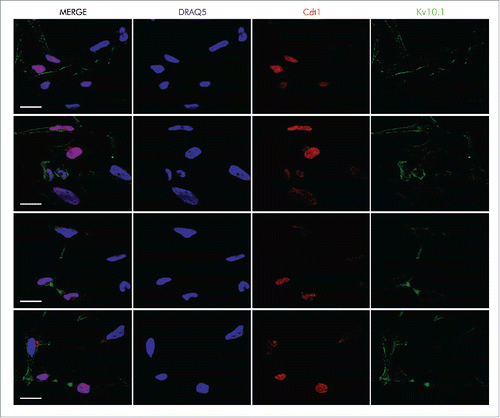
Figure 3. Kv10.1 expression in G2/M depends on pRb/E2F1 activity. A. Schematic of human Kv10.1 promoter indicating E2F1 responsive element upstream of the transcription starting site (TSS). B. Luciferase activity driven by Kv10.1 promoter (black trace) showed a peak activity during G2/M transition (One-Way ANOVA, P < 0.0001). Mutation of E2F1 responsive element (red trace) abolished promoter activity (Two-way ANOVA. Changes in expression levels with time after release P < 0.0001, mutated vs. wild type Kv10.1 promoter P < 0.0001). C. HPV-E7 overexpression increased Kv10.1 promoter activity (Two-way ANOVA. Changes in expression levels with time after release P < 0.01, effect of HPV-E7 overexpression P < 0.0001). D. Analysis by SDS-PAGE and Western blotting using anti-Kv10.1, anti-pRb and anti-E2F1 showed that Kv10.1 expression profile is delayed from E2F1 peak expression. E. E2F1 binding to endogenous Kv10.1 promoter (One-Way ANOVA, P < 0.0001) and Cyclin A2 promoter (One-Way ANOVA, P < 0.001) during G2/M transition (8 h). Cyclin A2, an E2F1 target gene regulated at the G2/M transition was used here as a positive control. Fold enrichment was calculated relative to GAPDH promoter signal. GAPDH is a non-E2F1 regulated gene. All experiments were performed at least 3 times.
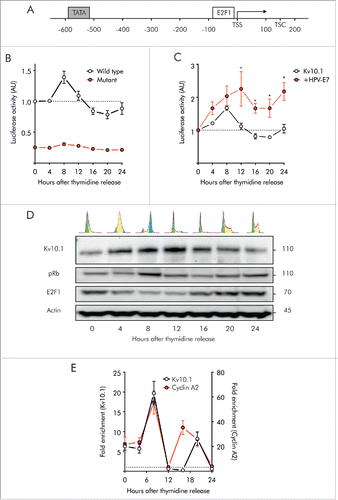
Figure 4. Kv10.1 depletion disrupts cell cycle progression in HeLa cells. A. Western blotting showed upregulated expression of Cyclins A2 and B1 upon Kv10.1 knockdown. Expression of Cyclins D1 and E1 was comparable between knockdown and control. B-D. HeLa cells transfected with siRNA control (black trace) and Kv10.1 siRNA (red trace) were synchronized with a double thymidine block and released into fresh medium. Cells were harvested at the indicated time points for FACS analysis of cell cycle profile. B. Percentage of cells in G0/G1 phase. C. Percentage of cells in S phase. D. Percentage of cells in G2/M phase. HeLa cells accumulated at G2/M upon Kv10.1 knockdown. All experiments were performed at least 3 times.
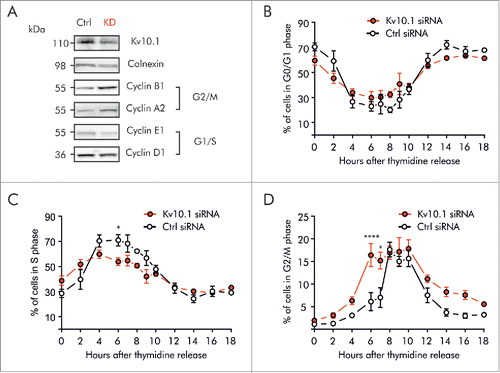
Figure 5. Kv10.1 depletion alters the periodicity of key G2/M regulatory proteins. HeLa cells were synchronized with a double thymidine block and released into fresh medium. Total cell lysates from siRNA control and Kv10.1 siRNA-transfected cells were prepared at the indicated time points. A. Analysis by SDS-PAGE and Western blotting of Cyclin D1 and E1 did not show differences. Levels of phosphorylated pRb rose at the same time in siRNA control and Kv10.1 siRNA transfected cells. However, pRb remained in hyperphosphorylated state for longer time in cells lacking Kv10.1. Densitograms from the blots corresponding to pRb Ser 780, pRb Ser 795 and pRb Ser 807–811 are plotted in the lower part of the panel. B. Western blotting showed that degradation of Cyclin A2 and B1 was delayed in Kv10.1 knockdown cells, as well as the dephosphorylation of pCdk1 (Y15). All experiments were performed at least 3 times.
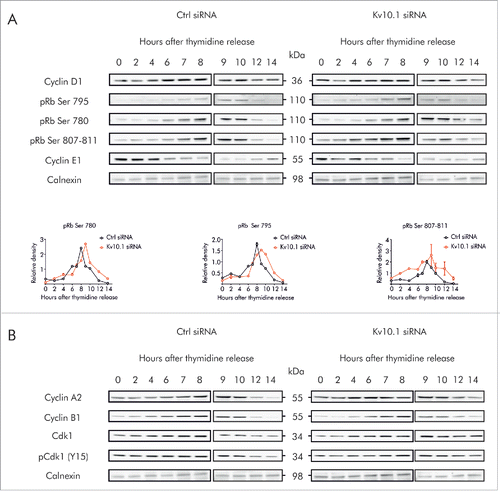
Figure 6. Kv10.1 absence alters cell cycle progression in mouse embryonic fibroblasts. A. Western blotting showed upregulated expression of Cyclins D1, A2 and B1 in Kv10.1 knockout MEFs. Expression of Cyclin E1 was comparable between knockout and wild type. (B-D) Wild type MEFs (black trace) and knockout MEFs (red trace) were synchronized after 72 h of serum starvation and released into fresh medium. MEFs were harvested at the indicated time points for FACS analysis of cell cycle status. B. Percentage of cells in G0/G1 phase. C. Percentage of cells in S phase. D. Percentage of cells in G2/M phase. Knockout MEFs accumulated at G2/M. All experiments were performed at least 3 times.
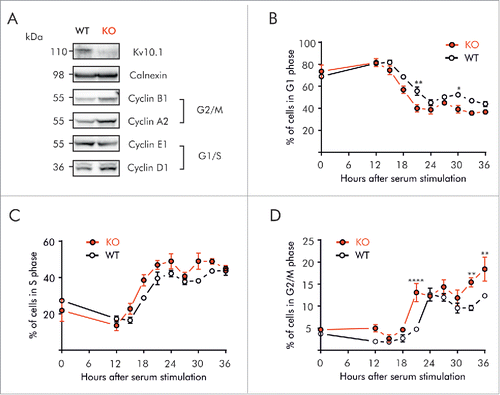
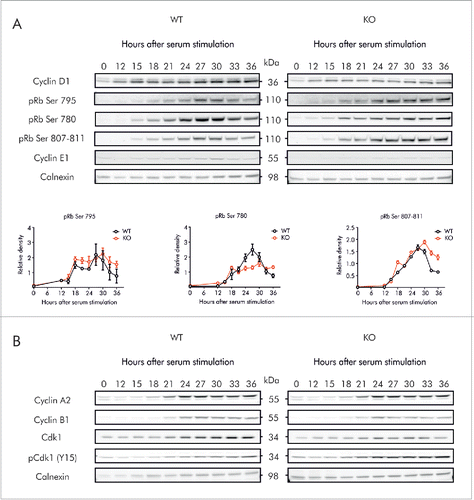
Figure 7. Kv10.1 absence alters the periodicity of key G2/M regulatory proteins. MEFs were synchronized after 72 h of serum starvation and released into fresh medium. Total cell lysates from wild type and Kv10.1 knockout MEFs were prepared at the indicated time points. A. Analysis by SDS-PAGE and Western blotting of Cyclin D1 and E1 did not show differences. Levels of phosphorylated pRb rose at the same time in wild type and Kv10.1 knockout MEFs. However, pRb remained in hyperphosphorylated state for longer time in cells lacking Kv10.1. Densitograms from the blots corresponding to pRb Ser 780, pRb Ser 795 and pRb Ser 807–811 are plotted in the lower part of the panel. B. Western blotting showed increased levels of the inhibitory phosphorylation (Y15) on Cdk1. All experiments were performed at least 3 times.