Figures & data
Figure 1. FMDV VP3 negatively regulates the IFN-γ-triggered signaling pathway. (A-B) Effects of the overexpression of FMDV proteins on IFN-γ -triggered IRF1 promoter activation. HEK293T cells (5×104) were transfected with the IRF1 reporter (0.1 μg), pRL-TK (as an internal control; 10 ng), and the indicated expression plasmids (0.1 μg). Twenty hours after transfection, the cells were treated with IFN-γ (50 ng/ml) or left uninfected for 12 h before luciferase assays were performed. The expression of FMDV proteins was analyzed by western blotting in B. (C) Dose-dependent effects of FMDV VP3 on the IFN-γ-triggered activation of the IRF1 promoter. The experiments were performed as described in A. (D) Effects of overexpression of FMDV VP3 on PMA-triggered AP-1 promoter activation. HEK293T cells (5×104) were transfected with the AP-1 reporter (0.1 μg), 10 ng of pRL-TK (as an internal control) and the indicated expression (0.1 μg) plasmids. Twenty hours after transfection, the cells were treated with phorbol 12-myristate 13-acetate (50 ng/ml) or left untreated for 12 h before luciferase assays were performed. (E) Increased copies of the FMDV genome in VP3-transfected BHK-21 cells. BHK-21 cells stably expressing VP3 or 3A were treated with IFN-γ (100 ng/ml) or left untreated for 1 h and infected with FMDV at the indicated times. FMDV genome copies were assessed using a real time quantitative RT-PCR assay. The values are expressed as the mean ± SD of three independent experiments. Protein expression was analyzed by protein gel blotting before treatment with IFN-γ . (F) VP3 localizes to the cytoplasm and nucleus in FMDV-infected BHK21 cells. BHK21 cells were infected with O-type FMDV (MOI=0.1) or left uninfected. The cells were fixed at 8 h post-infection (hpi) and subjected to an indirect immunofluorescence assay to detect the VP3 protein (green). The position of the nucleus is indicated by DAPI (blue) staining in the merged image. Rel. Luc. Act.: relative luciferase activity, EV: empty vector.
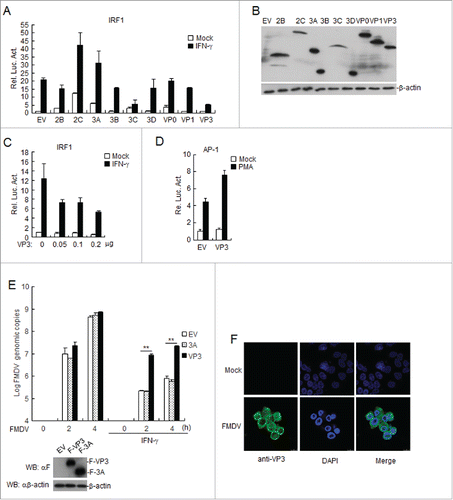
Figure 2. FMDV VP3 inhibits the IFN-γ-triggered induction of downstream genes. HEK293T cells were transfected with a plasmid encoding VP3 or an empty vector (EV) (0.5 μg) for 24 h. The cells were then treated with IFN-γ (100 ng/ml) for 3 h, and the IRF1, GBP1, STAT1, CXCL9, and GAPDH genes were evaluated using a relative quantitative RT-PCR assay. The values are presented as the mean ± SD of three independent experiments.
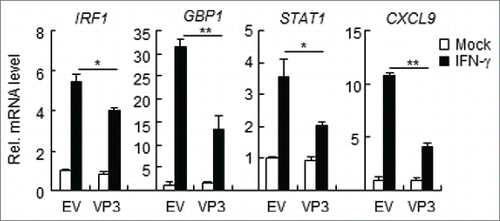
Figure 3. FMDV VP3 inhibits the phosphorylation, dimerization and nuclear accumulation of STAT1 induced by IFN-γ. (A) Western blotting was used to assess the effects of VP3 on the phosphorylation of STAT1 after IFN-γ (100 ng/ml) stimulation. HEK293T cells were transfected with pCAGGS-Myc-VP3 for 24 h and were left untreated or treated with IFN-γ at the indicated time points. Western blotting was used to analyze pY701-STAT1, STAT1, IRF1 or Myc-VP3 expression in the cell lysates. (B) Western blotting was also used to examine the effects of VP3 on the dimerization of STAT1. HEK293T cells were transfected with 4 μg of the Myc-VP3 plasmid (+) or an empty vector (−), 5 μg of HA-STAT1 and 5 μg of Flag-STAT1. Co-immunoprecipitation was performed with anti-HA (F) or control IgG (Ig) antibodies. Immunoblotting analysis was performed with anti-Flag antibody (αF) (upper panels). The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with antibodies specific for HA, Flag and Myc (lower panels). (C) The effects of VP3 on the dimerization of STAT1 after IFN-γ stimulation. HEK293T cells were transfected with pCAGGS-Myc-VP3 (4 μg) for 24 h and were left untreated or treated with IFN-γ (100 ng/ml) at the indicated time points. Western blotting was used to analyze pY701-STAT1, STAT1 and Myc-VP3 expression in the cell lysates via SDS-PAGE, and STAT1 monomer and dimer expression in the cell lysates was assessed via Native-PAGE. Densitometry analysis of the original western blots was performed using the Image J software. (D) FMDV VP3 blocks the nuclear accumulation of phosphorylated STAT1. HEK293T cells stably expressing VP3 were treated with IFN-γ (100 ng/ml) at the indicated time points. The cytoplasmic and nuclear proteins were extracted using the CelLytic nuclear extraction kit (catalog no. Nxtract; Sigma-Aldrich). Western blotting was used to analyze pY701-STAT1, STAT1 and F-VP3 expression in the cytoplasm, nucleus and whole-cell lysate. (E) FMDV VP3 blocks the nuclear accumulation of phosphorylated STAT1, as indicated by confocal immunofluorescence microscopy. HEK293T cells stably expressing VP3 were treated with IFN-γ (100 ng/ml) at the indicated time points. The cells were fixed and subjected to an indirect immunofluorescence assay to detect the phosphorylated STAT1 protein (red). The cell nucleus was counterstained with DAPI. The nuclear accumulation of phosphorylated STAT1 was observed using a Leica SP2 confocal system (Leica Microsystems). Protein expression was analyzed by protein gel blotting before treatment with IFN-γ. EV, empty vector.
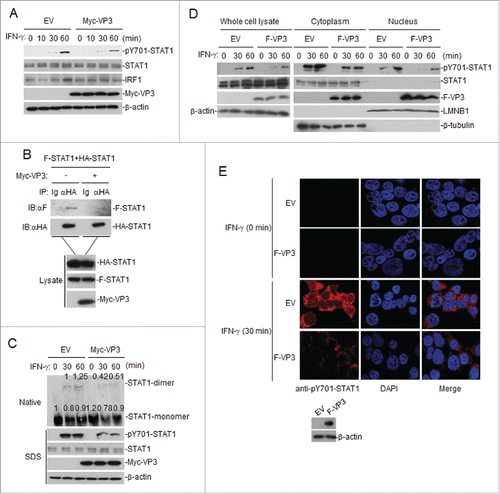
Figure 4. FMDV VP3 targets JAKs to regulate IFN-γ-triggered signaling. (A) Luciferase assays were used to assess how FMDV VP3 affects IRF1 activation through various signaling components. HEK293T cells were transfected with 100 ng of the IRF1 reporter, VP3 (100 ng), 10 ng of pRL-TK (as an internal control), and the indicated proteins (100 ng). Luciferase assays were performed 24 h after transfection. Western blotting was used to analyze JAK1, JAK2, and VP3 expression levels. (B-C) Dose-dependent effects of VP3 on JAK1- and JAK2-triggered activation of the IRF1 promoter. HEK293T cells were transfected with 100 ng of the IRF1 reporter, VP3, 10 ng of pRL-TK (as an internal control), and JAK1 or JAK2 (100 ng). Luciferase assays were performed 24 h after transfection. Western blotting was used to analyze JAK1, JAK2, and VP3 expression levels. (D-E) JAK1 and JAK2 increase the expression of the FMDV VP3 protein. HEK293T cells were co-transfected with increased amounts of JAK1 or JAK2 (0, 0.5, and 1.0 μg) and a constant quantity of VP3 (50 ng). At 24 h post-transfection, the cell lysate was analyzed by western blotting. The values are presented as the mean ± SD of three independent experiments.
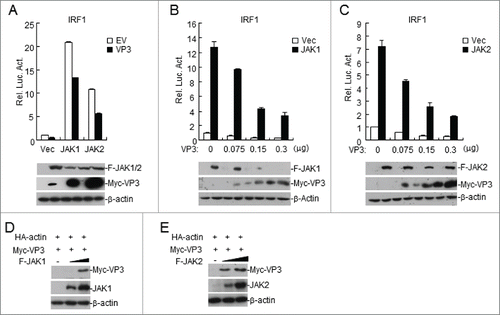
Figure 5. FMDV VP3 interacts with JAK1 and JAK2. (A) Interaction between FMDV VP3 and JAK1 or JAK2 in a mammalian overexpression system. The HEK293T cells (2×106) were co-transfected with 4 μg of the FMDV Myc-Vp3 plasmid and plasmids expressing Flag-JAK1 (8 μg), Flag-JAK2 (8 μg) or Flag-STAT1 (5 μg). Co-immunoprecipitation was performed with anti-Flag (F) or control IgG (lg) antibodies. Immunoblotting analysis was performed with anti-Myc-HRP (αMyc) (upper panels). The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with antibodies specific for Flag and Myc (lower panels). (B) FMDV VP3 interacts with endogenous JAK1. The HEK293T cells (1.6×107) were co-transfected with 20 μg of the FMDV Flag-VP3 (F-VP3) plasmid for 24 hours. Co-immunoprecipitation was then performed with anti-JAK1 or control IgG (lg) antibodies. Immunoblotting analysis was performed with anti-JAK1 or anti-Flag-HRP (αF) (upper panels) antibodies. The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with anti-JAK1 and anti-Flag (lower panels). (C) VP3 affects JAK1-STAT1 interactions. HEK293T cells were transfected with plasmids encoding Myc-VP3 (4 μg), Flag-JAK1 (8 μg), and HA-STAT1 (5 μg) for 24 h. Co-immunoprecipitation was then performed with anti-Flag (F) or control IgG (lg) antibodies. Immunoblotting analysis was performed with anti-HA or anti-Flag (upper panels) antibodies. The expression levels of the proteins were analyzed via immunoblotting analysis of the lysates with anti-Flag, anti-HA, and anti-Myc (lower panels). (D) VP3 does not affect JAK2-STAT1 interactions. The experiments were performed as described in C.
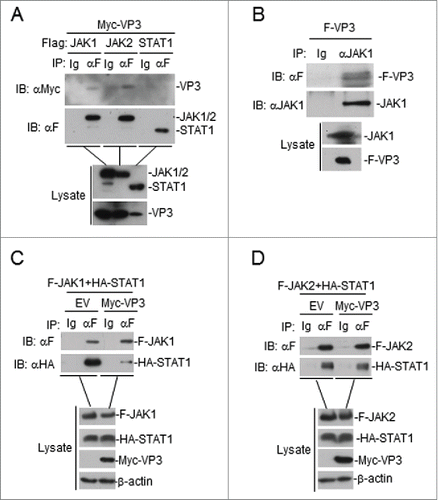
Figure 6. FMDV VP3 adversely affects JAK1 protein levels. (A-B) Dose-dependent effects of VP3 on JAK1 and JAK2. HEK293T cells were co-transfected with increased amounts of Myc-VP3 (0, 0.5, 1.0, and 2.0 μg) and JAK1 or JAK2 (200 ng). At 24 hours post-transfection, the cell lysate was analyzed by protein gel blotting. (C) Dose-dependent effects of FMDV 3A on JAK1. HEK293T cells were co-transfected with increased amounts of Flag-3A (0, 0.5, 1.0, and 2.0 μg) and a constant quantity of JAK1 (200 ng). At 24 hours post-transfection, the cell lysate was analyzed by western blotting. (D) Effects of FMDV VP3 on endogenous JAK1 and JAK2 expression. HEK293T cells stably expressing VP3 or 3A were lysed and analyzed by protein gel blotting. EV or minus: empty vector.
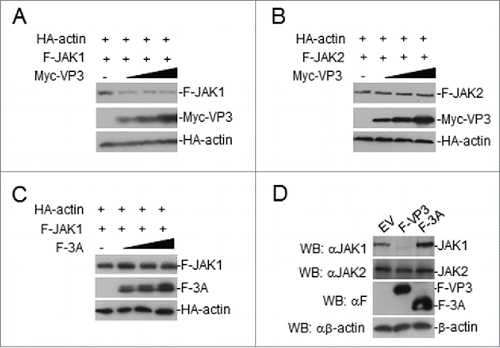
Figure 7. FMDV VP3 degrades JAK1 via a lysosomal pathway. (A-B) FMDV VP3 degrades JAK1 via a lysosomal pathway. HEK293T cells were transfected with VP3 (1.0 μg) and JAK1 (150 ng) for 18 h and then treated with dimethyl sulfoxide (DMSO), MG-132 (20 μM), 3-MA (0.5 mg/ml), NH4Cl (20 mM) or Leupeptin (400 μg/ml) for 6 h. The cell lysate was analyzed by western blotting. (C) Dose-dependent effects of VP3 on JAK1 mRNA expression levels. HEK293T cells were transfected with increasing amounts of FMDV VP3 for 24 h. The JAK1 gene was evaluated using a relative quantitative RT-PCR assay. The values are presented as the mean ± SD of three independent experiments.
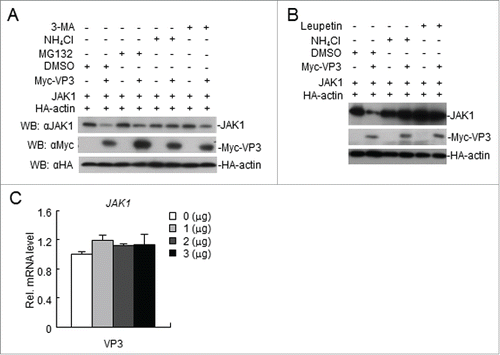
Table 1. Primers used in this study.
