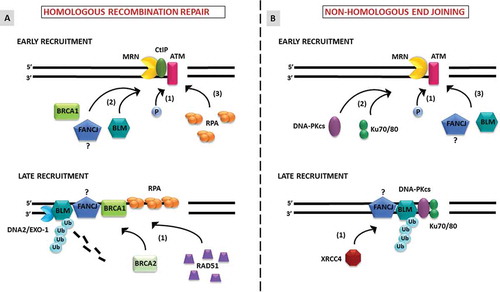
Figures & data
Table 1. Properties of the BLM and FANCJ DNA helicases.