Figures & data
Figure 1. Purification of soluble α6β4 proteins. (A) α6-Fcβ4 (top panel) and α6β4-Fc (bottom panel) proteins were purified from α6-Fcβ4– or α6β4-Fc–transfected CHO cell supernatants respectively by protein A chromatography. Protein purity in column fractions in the load (L), flow through (FT), wash (W), and elution fractions (1, 2, 3) was assessed by SDS-PAGE under nonreducing conditions. α6-Fc, β4, β4-Fc, and α6 bands at predicted sizes of 272, 77, 204, 110 kDa, respectively, are indicated. (B) The identity of the purified α6-Fcβ4 (top panel) or α6β4-Fc (bottom panel) material was confirmed by Western blotting under nonreducing conditions with antibodies to α6 and β4. (C) SEC and light scattering (top right inset; RI = refractive index, MW = molecular weight) analysis shows soluble α6-Fcβ4 migrating at the approximate size of a tetramer (449.4 kDa). Predicted size of two α6-Fc subunits plus two β4 subunits is 426 kDa. Inset (top left) is a cartoon of the α6-Fcβ4 tetramer.
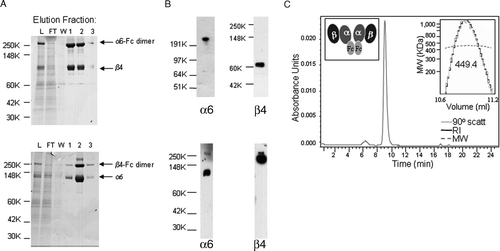
Figure 2. α6β4 antibodies recognize both soluble and endogenous proteins. (A) Plates were coated with the indicated proteins then incubated with α6- (4F10) or β4- (ASC-3 and ASC-8) specific antibodies. Antibody binding was detected using an HRP-conjugated anti-mouse antibody as described. (B–D) Binding characteristics of β4- or α6-specific antibodies were determined by flow cytometry on colorectal tumor cell lines SW620 (B) and SW480 (C), and breast tumor cell line MDA-MB-231 (D).
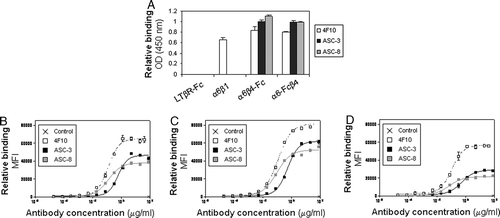
Figure 3. Ligand binding specificity of soluble α6β4 proteins is comparable to that of endogeous α6β4. (A, B) Purified α6-Fcβ4 (A) or α6β4-Fc (B) were incubated on plates coated with the indicated extracellular matrix proteins in the presence of 10 mM MgCl2 (grey and black bars), 1 mM MnCl2 (dashed lines), or 10 mM EDTA (white bars). After washing, bound α6-Fcβ4 or α6β4-Fc was detected using an HRP-conjugated anti-human antibody. (C) Colorectal tumor cells (SW480) were starved for 24 h in serum-free medium. Cells were held in suspension for 1 h on ice prior to plating on 96-well plates coated with the indicated matrices for 1 h at 4°C. Relative adhesion was measured using a luciferase-based luminescent viability assay.
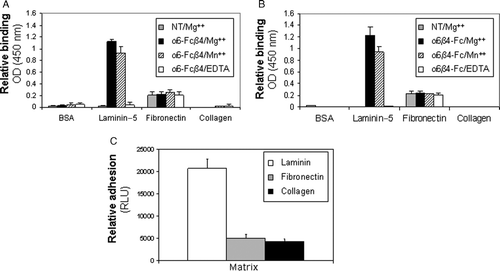
Figure 4. Effect of β4-specific antibodies on α6β4-laminin adhesion. (A) Purified α6-Fcβ4 or α6β4-Fc proteins were left untreated (NT; white bars), preincubated with a β3-specific control antibody (Anti-β3; dashed bars) or β4-specific antibodies (ASC-3; black bars, ASC-8; grey bars) prior to plating on laminin-5–coated ELISA plates. Bound α6β4 proteins were detected using an HRP-conjugated anti-human antibody. (B) Colorectal tumor cells (SW480) were starved for 24 h in serum-free medium. Cells were left untreated (NT; white bars), treated with a β3-specific control antibody (Anti-β3; dashed bars) or with β4-specific antibodies (ASC-3; black bars, ASC-8; grey bars) and held in suspension for 1 h on ice prior to plating on laminin-5–coated plates for 1 h at 4°C. Relative adhesion was measured using a luciferase-based luminescent viability assay.
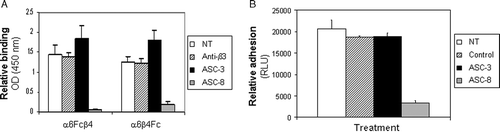
Figure 5. β4 antibodies recognize different epitopes on α6β4. (A) Biotinylated ASC-3 or ASC-8 were allowed to bind to α6-Fcβ4–coated plates preincubated with saturating concentrations of unlabeled antibodies for 1 h, then detected using avidin-HRP. (B) Purified β4 was obtained from α6-Fcβ4 as described and analyzed by SDS-PAGE under nonreducing conditions (lane 1: α6Fc-β4; lane 2: purified β4 protein). α6-Fc and β4 bands at predicted sizes of 272 and 77 kDa, respectively are indicated. (C) ASC-3 or ASC-8 (0.1 µg/ml) were incubated on α6-Fcβ4 (0.1 µg/ml)–coated plates in the presence of increasing amounts of β4 protein. Bound antibodies were detected using an HRP-conjugated anti-mouse antibody as described.
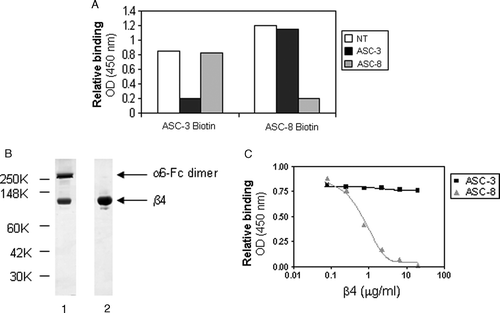
Figure 6. β4-specific antibodies exhibited different kinetic parameters by surface plasmon resonance. Concentrations of ASC-3 (A) or ASC-8 (B) ranging from 500 or 30 nM to 2 or 1.2 nM, respectively, were allowed to flow over an anti-human IgG1 coupled CM-5 chip saturated with α6-Fcβ4.
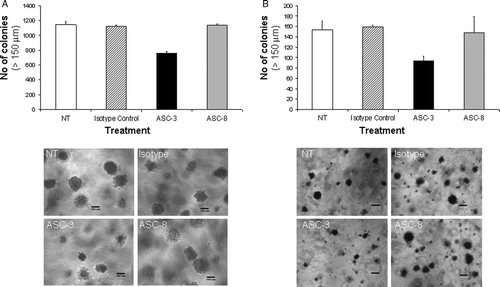
Figure 7. Effects of β4-specific antibodies on anchorage-independent growth. (A) Colon (SW620) or (B) breast (MDA-MB-231) cancer cells were grown in 0.3% soft agar containing ASC-3 or ASC-8 (10 µg/ml). Plates were supplemented twice weekly with antibody-containing medium. Anchorage-independent growth was scored by manual counting of colonies >150 µm 21 to 27 days after plating. Error bars represent variation between duplicates. Pictures of colonies are representative examples. Size bar represents 199 µm.