Figures & data
Table 1 Uses of Dosimetry Models and Methods in Quantitative Risk Assessment
Table 2 Hierarchy of Dosimetry Models and Methods. Ordered from Simpler, Less Specific Approaches to More Complex, Chemical-Specific Approaches.
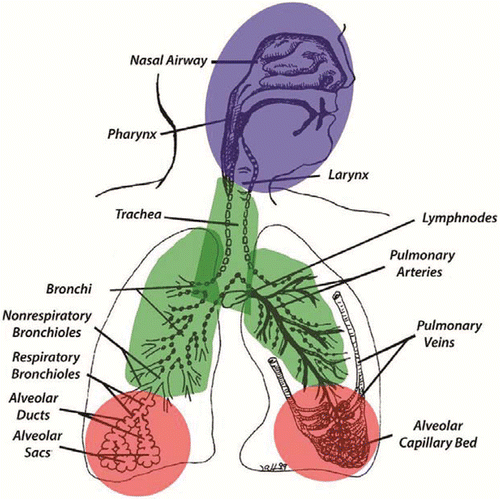
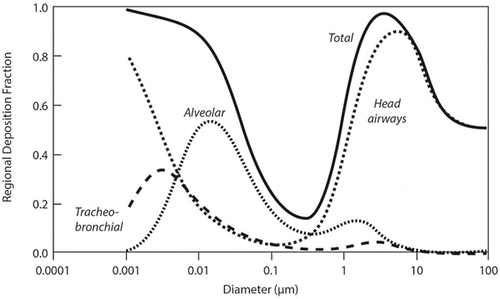
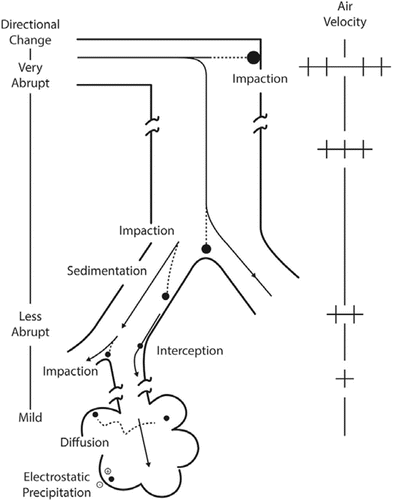
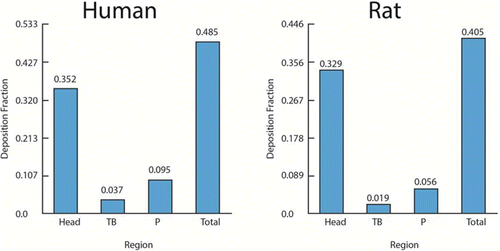
Table 3 Gas Categories and Characteristics(Citation4,Citation11)
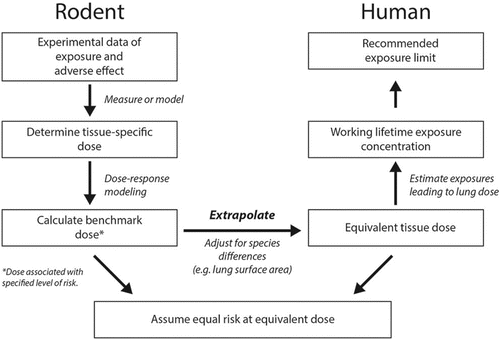
![Figure 6 Process for OSHA methylene chloride PEL development and risk estimate.(Citation112) In developing their final rule, OSHA used Bayesian analysis to fit their model to multiple pharmacokinetic data sets for mice and humans to arrive at estimates for posterior distributions of PBPK model parameter values. Using these posterior parameter distributions, estimates of the dose surrogates (production of metabolites via the glutathione-S-transferase [GST] pathway in the lung) produced in the key mouse bioassay were computed. OSHA conducted analyses using the human PBPK model with a baseline set of parameters for the GST pathway derived from the mouse values via allometric scaling and an alternative human GST pathway parameter set derived by incorporating human in vitro metabolism data using the parallelogram approach (as described in Reitz et al.(Citation169)). The mouse lung dose surrogates were used as inputs to derive the parameters for the linearized multistage cancer dose-response relationship. The human lung dose surrogates for the new PEL were then computed using the human PBPK model, and working lifetime cancer estimates derived from the 95th percent upper confidence limit of the baseline and alternative dose surrogates.](/cms/asset/35b61441-845d-4a6e-a36b-4638bd422024/uoeh_a_1060328_f0006_oc.gif)
Table 4 Examples of Available Tools and Resources for Dosimetry Modeling
Supplemental material
