Figures & data
FIGURE 1. Morphologic analysis and phenotypic identification of PDLSCs. (A) Morphology of PDLSCs which demonstrated spindle-like shape. Scale bar: 100 µm. (B) Cell surface antigens of PDLSCs at passage 2 were detected by flow cytometry. The percentages of cell surface CD markers were shown in the histograms.
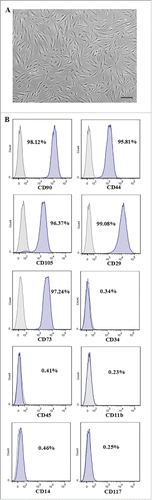
FIGURE 2. Effect of IL-7 on the proliferation of PDLSCs. PDLSCs were treated by 0, 10 and 20 ng/ml IL-7 for 24, 48, 72 and 96 h, followed by determination with MTT assays for cell proliferation. Data are the mean absorbance values at 570 nm of 3 experiments and the bars represented SD of the mean.
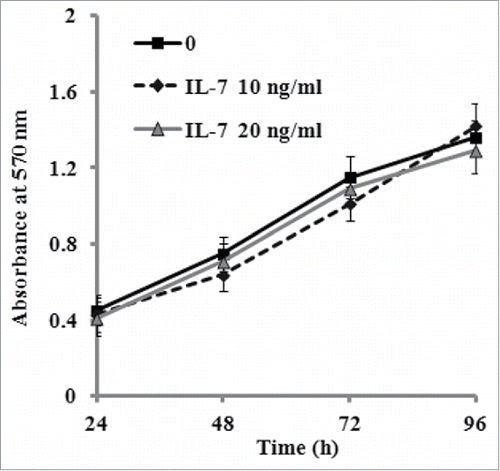
FIGURE 3. IL-7 suppresses osteogenic differentiation of PDLSCs. After osteogenic induction culture for 2 or 3 weeks with or without IL-7 (10 and 20 ng/ml), ALP activity and calcium deposition and extracellular matrix mineralization were evaluated, respectively. (A) ALP activity was quantified and expressed relative to the untreated cells, to which an arbitrary value of 1 was given. (B) Representative images of Alizarin red S staining. (C) Alizarin red S staining (B) was extracted and, the amount of released dye was then measured by a spectrophotometer at 562 nm for absorbance value. Data are presented as mean ± SD, *P < 0.05 and **P < 0.01 compared with the control (0 ng/ml of IL-7). #P < 0.05 compared with the group (10 ng/ml of IL-7).
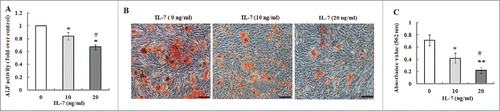
FIGURE 4. Effects of IL-7 on the expression levels of Runx-2, SP7 and OCN. (A-C) Gene expression of Runx-2, SP7 and OCN in PDLSCs was determined by qRT-PCR after osteogenic induction culture with 10 and 20 ng/ml of IL-7 for 2 weeks. (D) Representative Western blot images demonstrated that protein expression of Runx-7, SP7 and OCN in PDLSCs was measured by Western blotting after osteogenic induction with 10 and 20 ng/ml of IL-7 for 2 weeks of culture. (E) Relative protein levels of Runx-2, SP7 and OCN were quantified by the densitometry of each band normalized to β-actin signal. Data are presented as mean ± SD, *P < 0.05 and **P < 0.01 compared with the control (0 ng/ml of IL-7). #P < 0.05 compared with the group (10 ng/ml of IL-7).
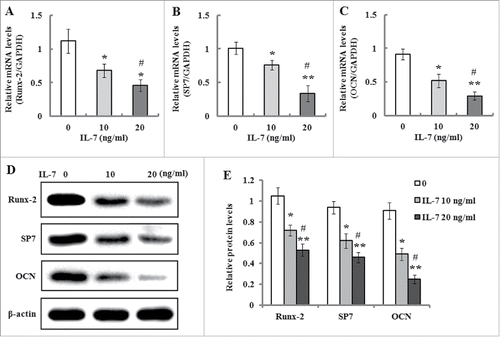
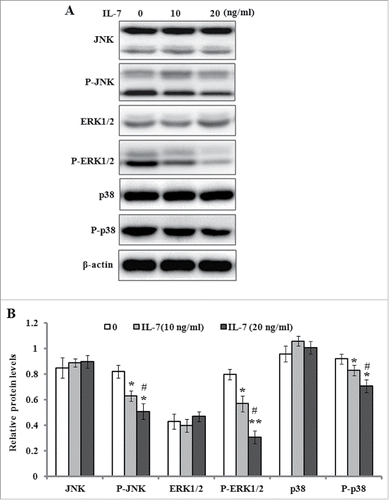
FIGURE 5. IL-7 inhibits the MAPK signaling in PDLSCs. PDLSCs were stimulated with IL-7 (0, 10 and 20 ng/ml) for 48 h, and total protein was extracted and subjected to Western blot analysis. (A) Representative western blot images demonstrating a markedly inhibitory role on the phosphorylation levels of JNK, ERK1/2 and p38, but not their protein levels. (B) Relative protein levels of JNK, ERK1/2, p38, and their phosphorylated levels were quantified by the densitometry of each band normalized to β-actin signal. Data are presented as mean ± SD, *P < 0.05 and **P < 0.01 compared with the control (0 ng/ml of IL-7). #P < 0.05 compared with the group (10 ng/ml of IL-7).