Figures & data
Figure 1. Innate immunity induction by mRNA vaccines. (a) Receptors of exogenous RNA and their signalling pathways. ppp-dsRNA: double stranded RNA possessing a triphosphate group at the 5’ end. (b) Potential mechanisms of innate immune responses to lipid components of iLNPs.
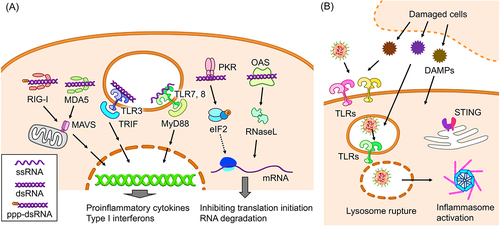
Table 1. Strategies to improve or modulate adjuvanticity in mRNA vaccines.
Figure 2. Strategies to improve adjuvanticity of mRNA vaccines. (a) iLNPs or other lipid systems. (b) A lipid carrier containing a calcium phosphate core. (c) Polyplexes. (d) Protamine/mRNA polyplexes. (e) A hollow-core sugar capsule coated with mannan. (F) Designing mRNA for improving adjuvanticity. Strategies and components to enhance adjuvanticity are highlighted in bold.
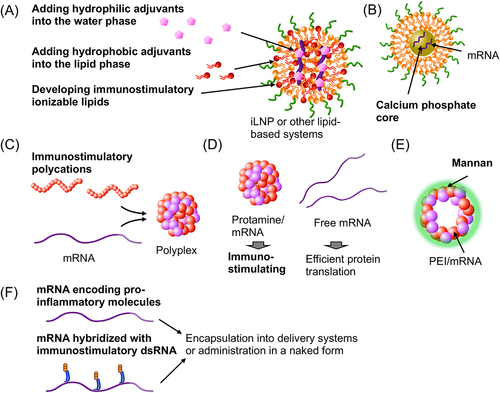
Figure 3. mRNA hybridized with dsRNA adjuvants. Translation efficiency and immunostimulatory properties after RNA hybridization are depicted. (a-c) Complementary RNA was hybridized to the coding region of mRNA (a, b) and poly a (c). (d) Comb-structured mRNA hybridized with dsRNA designed to target RIG-I in the coding region of mRNA.
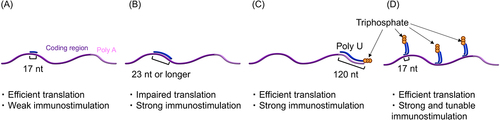
Figure 4. Effects of 1 mΨ modification on cancer vaccination. (a) iLNP loading ovalbumin (OVA) mRNA was intramuscularly injected into mice bearing subcutaneous B16F0 tumors expressing OVA. Tumor sizes are evaluated. (b) mCherry mRNA with 0, 40, 70, and 100% 1 mΨ modification was intramuscularly injected using iLNPs. The efficiency of mCherry expression in conventional type 1 and 2 dendritic cells (cDC1 and cDC2) in the lymph nodes and spleen was quantified. Reproduced from [Citation92] under a creative commons attribution license (CC BY). Copyright © 2022 sittplangkoon, Alameh, Weissman, Lin, Tam, Prompetchara and Palaga.
![Figure 4. Effects of 1 mΨ modification on cancer vaccination. (a) iLNP loading ovalbumin (OVA) mRNA was intramuscularly injected into mice bearing subcutaneous B16F0 tumors expressing OVA. Tumor sizes are evaluated. (b) mCherry mRNA with 0, 40, 70, and 100% 1 mΨ modification was intramuscularly injected using iLNPs. The efficiency of mCherry expression in conventional type 1 and 2 dendritic cells (cDC1 and cDC2) in the lymph nodes and spleen was quantified. Reproduced from [Citation92] under a creative commons attribution license (CC BY). Copyright © 2022 sittplangkoon, Alameh, Weissman, Lin, Tam, Prompetchara and Palaga.](/cms/asset/e32a675d-1b6f-47e1-8a36-134b5c2921f0/krnb_a_2333123_f0004_oc.jpg)
Figure 5. Effects of 1 mΨ modification on vaccines against infectious diseases. (a) Mice were intradermally vaccinated with unmodified or 1 mΨ-modified mRNA expressing hemagglutinin (HA) of the influenza virus using iLNPs. HA inhibition titer was evaluated. (b) Luciferase expression efficiency was evaluated after intradermal injection with iLNP loading unmodified and 1 mΨ modified mRNA. Reproduced from [Citation104] under a creative commons attribution license (CC BY-NC-SA). Copyright © 2018 Pardi et al.
![Figure 5. Effects of 1 mΨ modification on vaccines against infectious diseases. (a) Mice were intradermally vaccinated with unmodified or 1 mΨ-modified mRNA expressing hemagglutinin (HA) of the influenza virus using iLNPs. HA inhibition titer was evaluated. (b) Luciferase expression efficiency was evaluated after intradermal injection with iLNP loading unmodified and 1 mΨ modified mRNA. Reproduced from [Citation104] under a creative commons attribution license (CC BY-NC-SA). Copyright © 2018 Pardi et al.](/cms/asset/3105cf86-0204-4888-a409-a95ff13f6fa3/krnb_a_2333123_f0005_oc.jpg)
Figure 6. Influences of iLNP pre-injection on later innate and adaptive immune responses. (A-B) iLNP loading non-antigen mRNA (eGFP), empty iLNP (eLNP), or PBS was intradermally injected. Two weeks later, iLNP loading hemagglutinin (HA) was injected, followed by the evaluation of vaccination effects 2 weeks after the vaccination. (a) Experimental scheme. (b) HA-specific antibody production. (c-e) Two weeks after injection of iLNP loading non-antigen mRNA, mice were challenged with influenza virus or Candida albicans. (c) Experimental scheme. (d, e) viral load after the challenge with influenza virus (d) and Candida albicans (e). Reproduced from [Citation113] under a creative commons attribution license (CC BY). Copyright © 2021 Qin et al.
![Figure 6. Influences of iLNP pre-injection on later innate and adaptive immune responses. (A-B) iLNP loading non-antigen mRNA (eGFP), empty iLNP (eLNP), or PBS was intradermally injected. Two weeks later, iLNP loading hemagglutinin (HA) was injected, followed by the evaluation of vaccination effects 2 weeks after the vaccination. (a) Experimental scheme. (b) HA-specific antibody production. (c-e) Two weeks after injection of iLNP loading non-antigen mRNA, mice were challenged with influenza virus or Candida albicans. (c) Experimental scheme. (d, e) viral load after the challenge with influenza virus (d) and Candida albicans (e). Reproduced from [Citation113] under a creative commons attribution license (CC BY). Copyright © 2021 Qin et al.](/cms/asset/a0bdcd81-9977-4d50-b1aa-25ad050f5e2c/krnb_a_2333123_f0006_oc.jpg)
Figure 7. Plausible mechanisms of iLNP mRNA vaccines after their intramuscular injection. In one pathway, iLNPs migrate to the lymph nodes to provide antigen protein expression in APCs in the lymph nodes. In the other, immune cells infiltrate into the injection sites and migrate to the lymph nodes after taking up antigens.
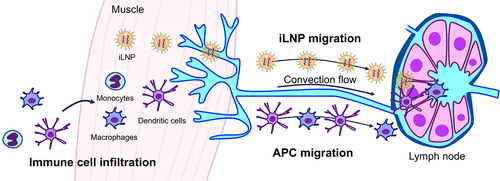
Table 2. Delivery strategies in mRNA vaccines.
Figure 8. Vaccination effects of different mRNA formulations. (a) mRNA vaccine formulations. (b) Humoral and cellular immunity induction of each vaccine formulation targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Reproduced from [Citation162] under a creative commons attribution license (CC BY-NC). Copyright © 2022 shi et al.
![Figure 8. Vaccination effects of different mRNA formulations. (a) mRNA vaccine formulations. (b) Humoral and cellular immunity induction of each vaccine formulation targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Reproduced from [Citation162] under a creative commons attribution license (CC BY-NC). Copyright © 2022 shi et al.](/cms/asset/1a013e49-033c-46a5-9410-37b31ee61224/krnb_a_2333123_f0008_oc.jpg)
Figure 9. Biodistribution of a lipopolyplex. (a) Preparation of the lipopolyplex. (b) Luciferase expression distribution after intramuscular injection of the lipopolyplex and iLNP loading luciferase mRNA. (c) mRNA distribution evaluated by qPCR after the lipopolyplex injection. A pie chart showed the cumulative mRNA copies in each organ. Reproduced from [Citation10] under a creative commons attribution license (CC BY). Copyright © 2021 Yang et al.
![Figure 9. Biodistribution of a lipopolyplex. (a) Preparation of the lipopolyplex. (b) Luciferase expression distribution after intramuscular injection of the lipopolyplex and iLNP loading luciferase mRNA. (c) mRNA distribution evaluated by qPCR after the lipopolyplex injection. A pie chart showed the cumulative mRNA copies in each organ. Reproduced from [Citation10] under a creative commons attribution license (CC BY). Copyright © 2021 Yang et al.](/cms/asset/c60fbcc9-68a6-4429-a1d0-aafa658a6e07/krnb_a_2333123_f0009_oc.jpg)
Figure 10. Roles of Langerhans cells (LCs) and conventional dendritic cell 1 (cDC1) in intradermal mRNA vaccination. mRNA expressing HA was intradermally injected using iLNPs. (A) Inhibition titer in wild-type (WT) mice or knockout mice lacking one or both of LCs and cDC1. (B) Antibody levels in WT mice injected with neutrophil-depleting antibodies (1A8) before vaccination. Reproduced from [Citation59] under a creative commons attribution license (CC BY). Copyright © 2022 Ndeupen et al.
![Figure 10. Roles of Langerhans cells (LCs) and conventional dendritic cell 1 (cDC1) in intradermal mRNA vaccination. mRNA expressing HA was intradermally injected using iLNPs. (A) Inhibition titer in wild-type (WT) mice or knockout mice lacking one or both of LCs and cDC1. (B) Antibody levels in WT mice injected with neutrophil-depleting antibodies (1A8) before vaccination. Reproduced from [Citation59] under a creative commons attribution license (CC BY). Copyright © 2022 Ndeupen et al.](/cms/asset/0169eaae-cfd5-43c1-a191-d121fe21ba8d/krnb_a_2333123_f0010_oc.jpg)
Figure 11. Targeting the human spleen using lipoplexes in systemic administration. 18F-2-deoxy-2-D-glucose positron emission tomography in a human after intravenous vaccination using anionic lipoplex. Spleens are encircled. Reproduced from [Citation190] under a creative commons attribution license (CC BY). Copyright © 2018 pektor et al.
![Figure 11. Targeting the human spleen using lipoplexes in systemic administration. 18F-2-deoxy-2-D-glucose positron emission tomography in a human after intravenous vaccination using anionic lipoplex. Spleens are encircled. Reproduced from [Citation190] under a creative commons attribution license (CC BY). Copyright © 2018 pektor et al.](/cms/asset/5f76ac93-64d8-41cd-b959-29556505eb2a/krnb_a_2333123_f0011_oc.jpg)
Figure 12. Targeting lymphoid tissues using polyplexes in mice. Reporter mRNA encoding luciferase (a) and tdTomato (b) was systematically injected. (a) Distribution of luciferase expression. (b) Cell types expressing tdTomato in the lymph nodes. Reprinted with permission from [Citation125]. Copyright © 2021 American Chemical Society.
![Figure 12. Targeting lymphoid tissues using polyplexes in mice. Reporter mRNA encoding luciferase (a) and tdTomato (b) was systematically injected. (a) Distribution of luciferase expression. (b) Cell types expressing tdTomato in the lymph nodes. Reprinted with permission from [Citation125]. Copyright © 2021 American Chemical Society.](/cms/asset/5d8d49b9-d5ea-4ddb-b257-1ce876e129b1/krnb_a_2333123_f0012_oc.jpg)
