Figures & data
Figure 1. MAPK11-MAPK14/p38-independent effects of SB202190/SB203580 in autophagy. (A) SB202190 and SB203580 (10 µM each) but not the other more specific MAPK11-MAPK14/p38 inhibitors (SB220025, 10 µM; BIRB-796, 1 µM; or VX-745, 10 µM) induce large vacuoles in HT29 cells (24 h treated). (B) The SB202190-induced vacuoles are acidic compartments as shown by strong acridine orange staining in primary HUVECs. (C) Autophagy inhibitor 3-MA suppresses SB-induced vacuolation in HT29 cells. (D) The efficacy of BIRB-796, SB202190, and SB203580 to inhibit MAPK14/p38α-MAPK11/p38β signaling in HeLa cells was compared by monitoring their effect on stress-induced phosphorylation of the direct MAPK14/p38α-MAPK11/p38β substrate MAPKAPK2 at Thr334 (T334) and of the downstream target HSPB1/HSP27 at Ser82 (S82). The membrane was reprobed with MAPKAPK2, HSPB1 and EEF2 (eukaryotic translation elongation factor 2) antibodies as loading controls. Cells were treated with the indicated concentrations of inhibitors (µM) prior to 30 min anisomycin (10 µg/ml) stimulation. (E and F) The off-target effect of SB202190 in autophagy is independent of cell-type specific vacuolation. In both, vacuole-positive HT29 and vacuole-negative HeLa cells (see ), long-term SB202190 treatment (10 µM for 4 or 24 h) leads to the accumulation of autophagy substrates SQSTM1 and lipid conjugated MAP1LC3B (LC3-II) (E). Quantified band intensities for LC3B-II and SQSTM1 normalized to that of the loading control (GAPDH) are shown (F). (G and H) Dose-dependent (10-30 µM) effect of SB203580 on autophagy in HeLa cells demonstrated by monitoring the levels of SQSTM1 and MAP1LC3B (LC3-II) at 24 h treatment (G). Quantified band intensities for lipid conjugated MAP1LC3B (LC3-II) and SQSTM1 normalized to the loading control (EEF2) are shown (H).
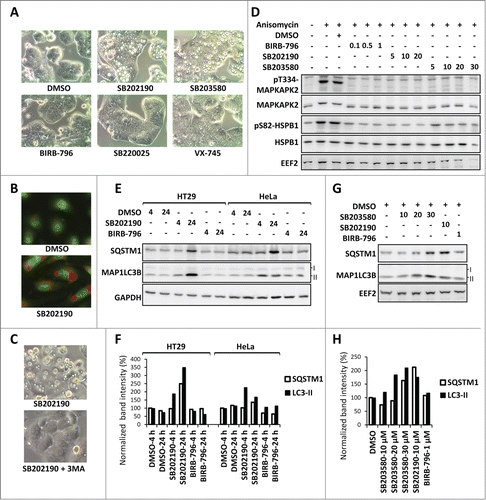
Table 1. Cell-type specificity of SB202190-induced vacuole formation.
