Figures & data
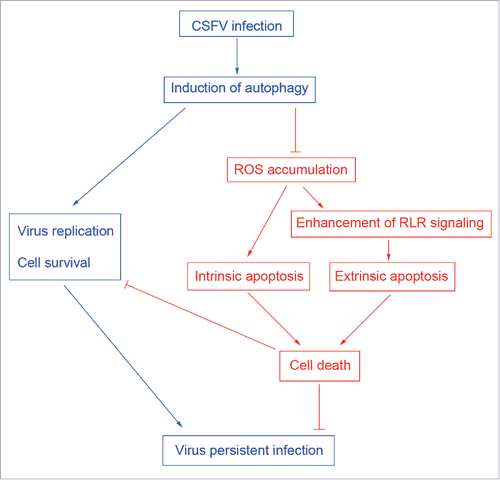
Figure 1. Rapamycin and autophagy-specific shRNAs treatments alter autophagic activity in host cells. (A) PK-15 cells were treated with rapamycin (100 nM) or DMSO (Mock) for 48 h as control groups. Other groups were infected with CSFV or UV-CSFV for 48 h at an MOI of 1. Then cells were fixed and processed for fluorescence signal detection of LC3 accumulation with corresponding antibodies as described in Materials and Methods. The fluorescence signals were visualized with confocal immunofluorescence microscopy. In images, LC3 staining is shown in green. Scale bar: 10 μm. Average number of punctate LC3 in each cell were determined from at least 100 cells in each group. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. **, P < 0.01; ***, P < 0.001; #, P > 0.05. Meanwhile, at the end of the infection, some cell samples were analyzed by immunoblotting with specific antibodies as described in Materials and Methods. Similar results were obtained in 3 independent experiments. (B) PK-15 cells were transfected with shRNAs targeting BECN1 or LC3B or scramble shRNA for 48 h, followed by mock infection and CSFV infection for 48 h at an MOI of 1. Then the cells were treated and analyzed as in (A). The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; ***, P < 0.001. Meanwhile, after infection, some cell samples were analyzed by immunoblotting with specific antibodies as described in Materials and Methods. Similar results were obtained in 3 independent experiments.
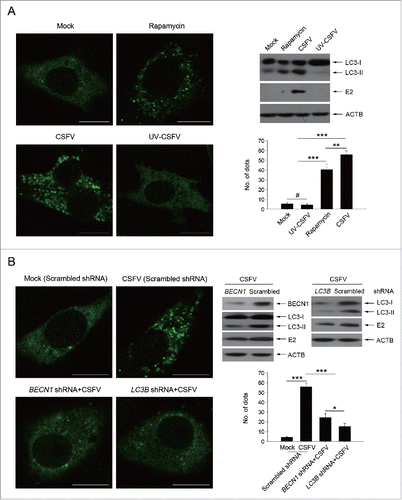
Figure 2. Alteration of autophagic activity affects viability of CSFV-infected cells. (A and C) PK-15 (A) and 3D4/2 (C) cells were pretreated with rapamycin (100 nM) or DMSO for 1 h. Cells were then mock infected or infected with CSFV at an MOI of 1. After 1 h of virus absorption at 37°C, the cells were incubated in fresh medium containing rapamycin (100 nM ) or DMSO for 0, 12, 24 and 48 h. Cells only treated with rapamycin were considered as one control. Meanwhile, 2 cell lines were transfected with shRNAs targeting BECN 1 or LC3B or scramble shRNA for 48 h, followed by mock infection and CSFV infection for 0, 12, 24 and 48 h at the same MOI. Then the cell viability was determined by the CCK-8 assay. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. (B and D) PK-15 (B) and 3D4/2 (D) cells were pretreated and transfected as described in (A and C), followed by mock infection and CSFV infection for 48 h at an MOI of 1. Percentage of cell death was then detected using a live-dead cell staining solution and analyzed by flow cytometry. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. **, P < 0.01; ***, P < 0.001; #, P > 0.05.
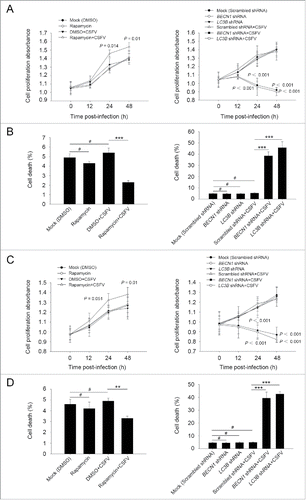
Figure 3. CSFV infection in autophagy knockdown cells induces apoptotic cell death in PK-15 cells. (A) PK-15 cells were transfected with the scrambled or BECN1 or LC3B shRNA for 48 h. Cells were then mock infected or infected with CSFV at an MOI of 1. After 1 h of CSFV absorption, the cells were further cultured in fresh medium in the absence or presence of Z-VAD (10 μM) or Nec-1 (10 μM) at the indicated time points. Then the cell viability was measured by the CCK-8 assay. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. (B) PK-15 cells were transfected and infected as described in (A). Percentage of cell death was then measured using a live-dead cell staining solution and assayed by flow cytometry. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P< 0.05; ***, P < 0.001; #, P > 0.05. (C) PK-15 cells transfected with the targeted shRNAs were incubated with CSFV at an MOI of 0.5, and were further cultured as described in (A). At 48 h after CSFV infection, the copy number of virus was detected by qRT-PCR as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. ***, P < 0.001; #, P > 0.05. (D) PK-15 cells were transfected and infected as described in (A). Apoptosis was then measured using a TUNEL Apoptosis Assay Kit as described in Materials and Methods. In the images, apoptotic cells are shown in green. Scale bar: 400 μm. Average number of apoptotic green “dots” was determined from at least 3 images. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. ***, P < 0.001; #, P > 0.05.
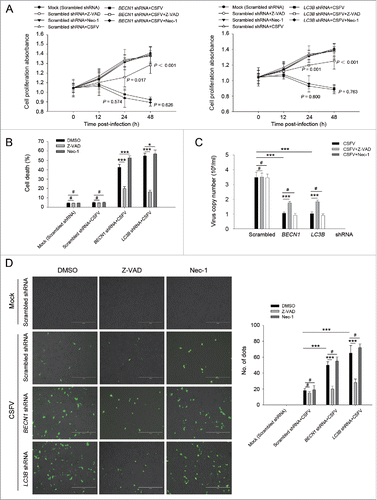
Figure 4. Autophagy knockdown promotes apoptosis in 3D4/2 cells under CSFV infection. (A) 3D4/2 cells were transfected and infected as described in the legend to . After 1 h of CSFV absorption, the cells were further cultured in fresh medium in the absence or presence of Z-VAD (10 μM) and Nec-1 (10 μM) at the indicated time points. Then the cell viability was measured by the CCK-8 assay. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. (B) 3D4/2 cells were treated as described in (A). Cell death was then detected using a live-dead cell staining solution and assayed by flow cytometry. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P< 0.05; ***, P < 0.001; #, P > 0.05. (C) 3D4/2 cells transfected with the autophagy-specific shRNAs were incubated with CSFV at an MOI of 0.5, and were further cultured as described in (A). At 48 h after CSFV infection, virus production was measured by qRT-PCR as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; #, P > 0.05. (D) 3D4/2 cells were treated as described in (A). Apoptosis was then measured using a TUNEL Apoptosis Assay Kit as described in Materials and Methods. In images, apoptotic cells are shown in green. Scale bar: 400 μm. Average number of apoptotic green “dots” were from at least 3 images. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. ***, P < 0.001; #, P > 0.05.
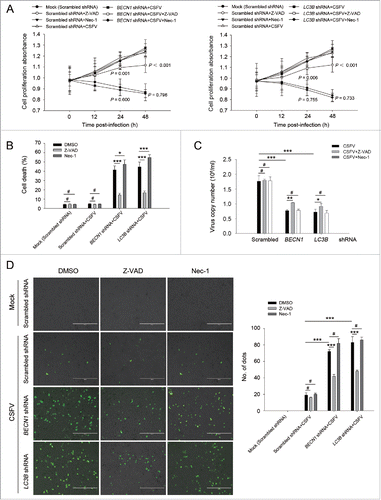
Figure 5. Autophagy deficiency leads to the activation of apoptosis pathways in CSFV-infected cells. ((A)and C) PK-15 (A) and 3D4/2 (C) cells were transfected and infected as described in the legend to . At 48 h after infection, the activity of CASP3, CASP8, and CASP9 in cells was measured by the CASP activity assay as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; #, P > 0.05. ((B)and D) PK-15 (B) and 3D4/2 (D) cells were transfected and infected as described in the legend to . After 48 h, the expression of BCL2, BAX, cleaved-CASP3, cleaved-CASP8, cleaved-CASP9, cleaved-PARP, and ACTB (loading control) were analyzed by western blot with specific antibodies as described in Materials and Methods. The relative expression ratios of these proteins were analyzed by densitometric scanning. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; ***, P < 0.001; #, P > 0.05.
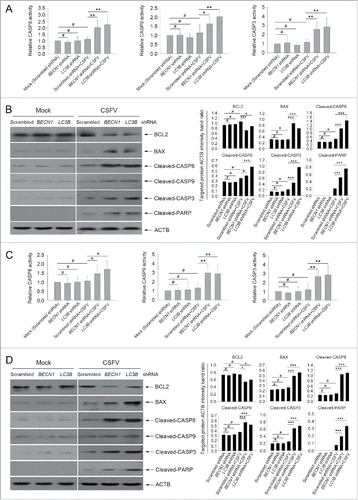
Figure 6. CSFV infection enhances the expression of IFN and ISGs in autophagy-deficient cells. (A) PK-15 and 3D4/2 cells were transfected and infected as described in the legend to . At 48 h after CSFV infection, the mRNA levels of IFNA, IFNB1, TNFSF10, TNFRSF10A, FASLG, FAS, TNF, and TNFRSF1A were detected by qRT-PCR as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P< 0.05; **, P < 0.01; ***, P < 0.001; #, P > 0.05. (B) PK-15 and 3D4/2 cells were transfected and infected as described in the legend to . After 48 h, the production of IFNA, IFNB1, and TNF was assessed by the ELISA assay. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; #, P > 0.05.
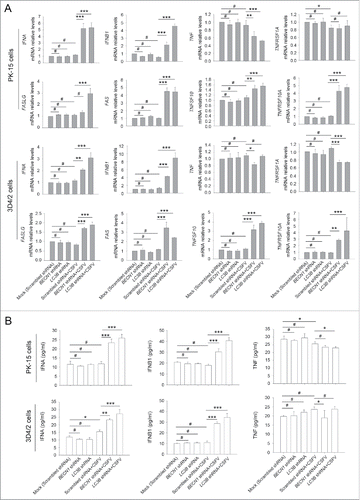
Figure 7. Type I IFN production is required for the activation of the extrinsic apoptosis pathway. (A) PK-15 and 3D4/2 cells were transfected and infected as described in the legend to , which were considered as the control groups. For inhibition of IFNA and IFNB1 induction, cells were cultured with bufexamac (10 μM) after 1 h of virus absorption, or cotransfected with shRNA targeting IFNB1 prior to CSFV infection. At 48 h after infection, the mRNA levels of TNFSF10, TNFRSF10A, FASLG, and FAS were measured by qRT-PCR. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; #, P > 0.05. (B) PK-15 and 3D4/2 cells were treated as described in (A). After 48 h, the activity of CASP3, CASP8, and CASP9 in cultured cells was detected by the CASP activity assay as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; #, P > 0.05. (C) PK-15 and 3D4/2 cells were treated as described in (A). After 48 h, cell samples were analyzed by western blot with antibodies against PARP and ACTB (loading control). The relative expression ratios of the targeted proteins were analyzed by densitometric scanning. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. ***, P < 0.001. (D) PK-15 and 3D4/2 cells were treated as described in (A). After 48 h, IFNA and IFNB1 production and the inhibition efficiency of type I IFN induction were detected by the ELISA assay. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; #, P > 0.05.
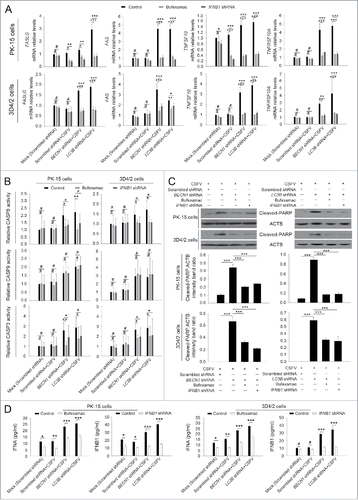
Figure 8. Absence of autophagy in CSFV-infected cells promotes the production of type I IFN by upregulating the RLR signaling. (A and B) PK-15 and 3D4/2 cells were transfected and infected as described in the legend to . At 48 h, the expression of DDX58, IFIH1, MAVS, and ACTB (loading control) were analyzed by western blot with specific antibodies as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P< 0.05; **, P < 0.01; ***, P < 0.001; #, P > 0.05. (C) PK-15 and 3D4/2 cells were cotransfected with the indicated shRNAs for 48 h, and were then infected with CSFV at an MOI of 1. At 48 h after infection, IFNA and IFNB1 production was detected by the ELISA assay. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; ***, P< 0.001; #, P > 0.05. (D) PK-15 cells were transfected with shRNAs targeting DDX58, IFIH1, or MAVS for 48 h, followed by CSFV infection at an MOI of 1. After 48 h, the expression of the targeted proteins was detected by western blot using anti-DDX58, anti-IFIH1, and anti-MAVS antibodies. Similar results were obtained in 3 independent experiments.
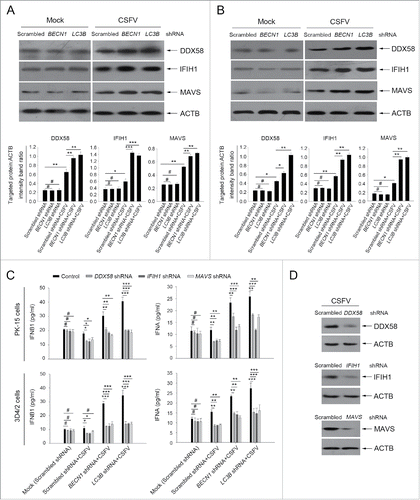
Figure 9. CSFV infection increases ROS levels in autophagy knockdown cells. (A) PK-15 and 3D4/2 cells were transfected with the scrambled, BECN1 or LC3B shRNAs for 48 h. After 1 h of mock infection or infection by CSFV at an MOI of 1, the cells were further cultured in fresh medium in the absence or presence of NAC (10 mM) or rotenone (1 μM) for 24 and 48 h. Levels of ROS in cultured cells were analyzed by MitoSOX staining as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P< 0.05, Dunnett's T3 (3) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01. (B) PK-15 and 3D4/2 cells were transfected and infected as described in the legend to . After 48 h, the copy number of mitochondrial DNA was assessed by qPCR as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. **, P < 0.01; ***, P < 0.001; #, P > 0.05.
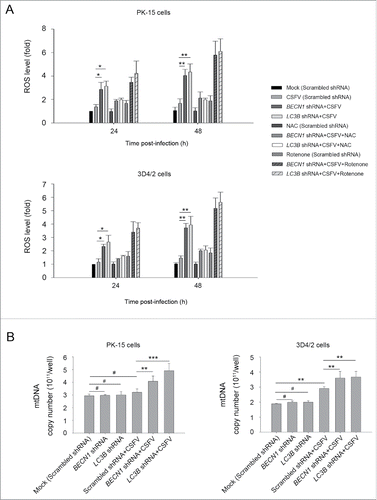
Figure 10. Alteration of ROS levels affects RLR signaling in cultured cells. (A) PK-15 and 3D4/2 cells were transfected with the BECN1 or LC3B shRNAs for 48 h. After 1 h of mock infection or infection by CSFV at an MOI of 1, the cells were further cultured in fresh medium in the absence or presence of NAC (10 mM) for 48 h. The expression of DDX58, IFIH1, and ACTB (loading control) were analyzed by western blot using specific antibodies. The relative expression ratios of these proteins were analyzed by densitometric scanning. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P< 0.05; **, P < 0.01; ***, P < 0.001. (B) PK-15 and 3D4/2 cells were transfected and infected as described in (A). After 1 h of CSFV infection at an MOI of 1, the cells were further cultured in fresh medium in the absence or presence of rotenone (1 μM) for 48 h. The expression of DDX58, IFIH1, and ACTB (loading control) were analyzed by western blot using specific antibodies. The relative expression ratios of these proteins were analyzed by densitometric scanning. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
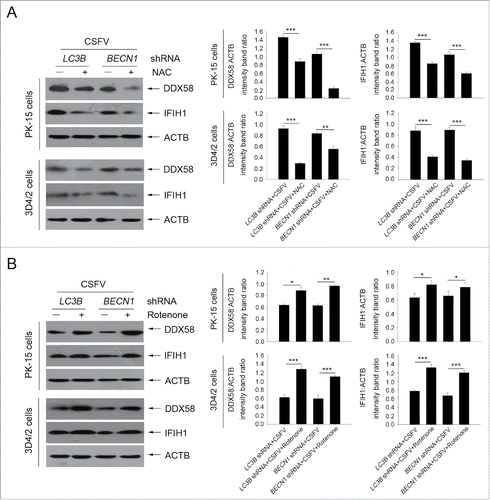
Figure 11. Regulation of ROS levels within autophagy-impaired cells triggers the extrinsic apoptosis pathway during CSFV infection. (A) PK-15 and 3D4/2 cells were treated and cultured as described in the legend to . The activities of CASP3, CASP8, and CASP9 in cell samples were assessed by the CASP activity assay as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P < 0.05, Dunnett's T3 (3) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; #, P > 0.05. (B) PK-15 and 3D4/2 cells were treated and cultured as described in the legend to . The levels of cleaved-PARP and ACTB (loading control) were detected by western blot using specific antibodies. The relative expression ratios of these proteins were assessed by densitometric scanning. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) PK-15 and 3D4/2 cells were transfected as described in the legend to . At 24 and 48 h after CSFV infection, virus copy number was detected by qRT-PCR as described in Materials and Methods. The data represent the mean ± SD of 3 independent experiments. One-way ANOVA test; test of homogeneity of variances, P > 0.05, LSD (L) was used for correction of post-hoc test. **, P < 0.01; ***, P < 0.001.
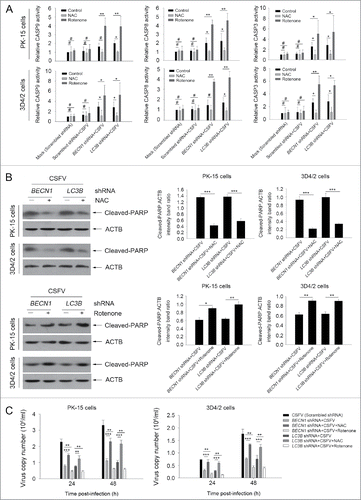
Table 1. shRNA sequences of targeted genes.
Table 2. Primers used in this study.