Figures & data
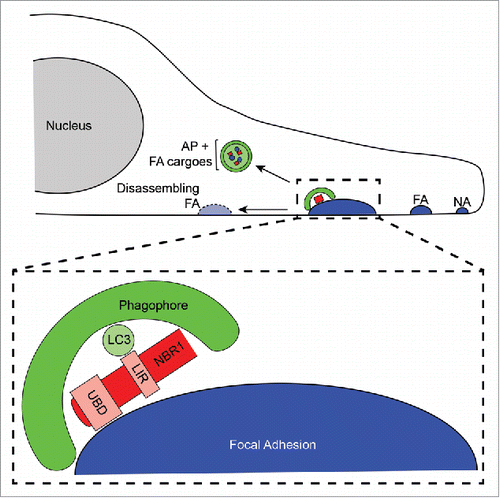
Figure 1. Selective autophagy promotes focal adhesion disassembly during cell migration. Forward, leading edge protrusions in migrating cells are stabilized by the formation of nascent adhesions (NA), which bind the extracellular matrix. NAs grow into mature, stable FAs through the addition of numerous scaffolding and signalling proteins. Subsequently, FAs must disassemble to allow the cell body to productively move forward, and NBR1-dependent selective autophagy is required for this process. NBR1 interacts with FAs via its ubiquitin binding domain (UBD), and this binding enables targeting of phagophores to FAs through interaction of the LC3 interacting region (LIR) of NBR1 with LC3 on the phagophore membrane. Autophagic targeting of FAs results in sequestration of FA proteins within autophagosomes (AP) and consequent destabilization of FAs leading to their disassembly.