Figures & data
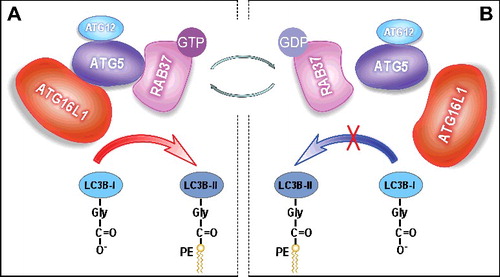
Figure 1. RAB37 regulates autophagosome formation by modulating ATG12–ATG5-ATG16L1 complex assembly. RAB37 as a key organizer participates in the process of ATG12–ATG5-ATG16L1 complex formation and promotes autophagy upon starvation induction in a GTP-dependent manner. RAB37, as a novel GTPase, regulates membrane functions through a switch between 2 distinct conformations: the GTP-bound “on” and the GDP-bound “off” forms. (A) RAB37-GTP interacts directly with ATG5 and promotes interaction of the ATG12–ATG5 conjugate with ATG16L1. The multimeric RAB37- ATG12–ATG5-ATG16L1 complex recruits and lipidates LC3B-I to form active LC3B-II, which accelerates autophagosomal formation. (B) RAB37-GTP can transform into the inactive RAB37-GDP probably through a GTPase activating protein. RAB37-GDP impairs its interaction with ATG5 and disassociates from ATG12–ATG5, then leads to separation of ATG12–ATG5 from ATG16L1. Finally, LC3B-I lipidation is prevented. Simultaneously, RAB37-GDP spreads to the cytosol for recycling via a guanine nucleotide exchange factor to organize another initiation process of autophagosomal formation upon autophagy induction.