Figures & data
Figure 1. The core autophagy machinery. Autophagy-related genes regulate autophagy [Citation8,Citation10], which is divided into four distinct steps: (1) initiation of autophagy by cellular stress, (2) phagophore nucleation, (3) phagophore elongation and closure, and (4) autophagosome fusion with the lysosome for the degradation and recycling of intravesicular material. Initiation of autophagy is predominantly mediated by the ULK1 complex with multiple regulatory subunits including ATG13 and RB1CC1. This complex is negatively regulated by MTORC1 through hyper-phosphorylation of ULK1 in nutrient-rich environments, whereas AMPK binds and phosphorylates ULK1 to activate autophagy under oxygen- and nutrient-deprivation [Citation200,Citation201]. Hypoxia induces autophagy through upregulation of HIF1A, which activates autophagy through BNIP3, AMPK, and EIF2AK3 signaling. Upon activation, ULK induces phagophore nucleation by phosphorylating the BECN1 complex, which consists of BECN1, ATG14 and PtdIns3K. BECN1 acts as a nexus point between autophagy, hypoxia, endosomal, and cell death pathways [Citation202]. Several effector molecules, including BNIP3, BCL2, BCL2L1/BCL-XL, UVRAG, RUBCN, and SH3GLB1/BIF-1, modulate BECN1 to positively and negatively regulate PtdIns3K activity [Citation202,Citation203]. Activation of PtdIns3K generates phosphatidylinositol-3-phophate (PtdIns3P) on the phagophore, which subsequently recruits other autophagy-related proteins to begin the process of elongation. Phagophore elongation is carried out by 2 ubiquitin-like conjugation processes. The first involves the formation of the ATG12–ATG5-ATG16L1 complex, which is mediated by the E1-like enzyme ATG7 and the E2-like enzyme ATG10 [Citation204]. The second involves the cleavage of LC3 by the protease ATG4B to make LC3-I, which is conjugated to phosphatidylethanolamine (PE) on the growing phagophore membrane by ATG7, the E2-like enzyme ATG3, and the E3-like ATG12–ATG5-ATG16L1 complex, generating LC3-II (LC3–PE). After completion of the autophagosome, the SNARE protein STX17 facilities fusion of the autophagosome with the lysosome, generating a hybrid vesicle called an autolysosome. Hydrolytic enzymes degrade the inner autophagosome membrane and its cargo, and the degradation products are released from lysosomes and recycled into metabolic and biosynthetic pathways.
![Figure 1. The core autophagy machinery. Autophagy-related genes regulate autophagy [Citation8,Citation10], which is divided into four distinct steps: (1) initiation of autophagy by cellular stress, (2) phagophore nucleation, (3) phagophore elongation and closure, and (4) autophagosome fusion with the lysosome for the degradation and recycling of intravesicular material. Initiation of autophagy is predominantly mediated by the ULK1 complex with multiple regulatory subunits including ATG13 and RB1CC1. This complex is negatively regulated by MTORC1 through hyper-phosphorylation of ULK1 in nutrient-rich environments, whereas AMPK binds and phosphorylates ULK1 to activate autophagy under oxygen- and nutrient-deprivation [Citation200,Citation201]. Hypoxia induces autophagy through upregulation of HIF1A, which activates autophagy through BNIP3, AMPK, and EIF2AK3 signaling. Upon activation, ULK induces phagophore nucleation by phosphorylating the BECN1 complex, which consists of BECN1, ATG14 and PtdIns3K. BECN1 acts as a nexus point between autophagy, hypoxia, endosomal, and cell death pathways [Citation202]. Several effector molecules, including BNIP3, BCL2, BCL2L1/BCL-XL, UVRAG, RUBCN, and SH3GLB1/BIF-1, modulate BECN1 to positively and negatively regulate PtdIns3K activity [Citation202,Citation203]. Activation of PtdIns3K generates phosphatidylinositol-3-phophate (PtdIns3P) on the phagophore, which subsequently recruits other autophagy-related proteins to begin the process of elongation. Phagophore elongation is carried out by 2 ubiquitin-like conjugation processes. The first involves the formation of the ATG12–ATG5-ATG16L1 complex, which is mediated by the E1-like enzyme ATG7 and the E2-like enzyme ATG10 [Citation204]. The second involves the cleavage of LC3 by the protease ATG4B to make LC3-I, which is conjugated to phosphatidylethanolamine (PE) on the growing phagophore membrane by ATG7, the E2-like enzyme ATG3, and the E3-like ATG12–ATG5-ATG16L1 complex, generating LC3-II (LC3–PE). After completion of the autophagosome, the SNARE protein STX17 facilities fusion of the autophagosome with the lysosome, generating a hybrid vesicle called an autolysosome. Hydrolytic enzymes degrade the inner autophagosome membrane and its cargo, and the degradation products are released from lysosomes and recycled into metabolic and biosynthetic pathways.](/cms/asset/20bc6371-6952-48a5-af71-e95c9aa66cc1/kaup_a_1450020_f0001_c.jpg)
Figure 2. Comparison of publications reporting autophagy is a metastasis promoting [Citation27,Citation28,Citation32,Citation59,Citation83,Citation92,Citation109,Citation125,Citation166,Citation205–210] or suppressing [Citation35,Citation72,Citation75,Citation76,Citation79,Citation93,Citation167,Citation169, Citation170,Citation211–215] mechanism, or both [Citation199]. The number of papers that include an in vivo mouse model is also included. Only publications explicitly reporting whether autophagy promotes or suppresses migration or metastasis were included.
![Figure 2. Comparison of publications reporting autophagy is a metastasis promoting [Citation27,Citation28,Citation32,Citation59,Citation83,Citation92,Citation109,Citation125,Citation166,Citation205–210] or suppressing [Citation35,Citation72,Citation75,Citation76,Citation79,Citation93,Citation167,Citation169, Citation170,Citation211–215] mechanism, or both [Citation199]. The number of papers that include an in vivo mouse model is also included. Only publications explicitly reporting whether autophagy promotes or suppresses migration or metastasis were included.](/cms/asset/fa891e4b-81a8-4f49-9e2c-1962d4e06e2a/kaup_a_1450020_f0002_c.jpg)
Figure 3. Autophagy regulates multiple metastasis-related signaling pathways. (A) Autophagy mediates the degradation of focal adhesion proteins to promote focal adhesion disassembly and migration. The autophagy protein LC3-II mediates the targeted degradation of several focal adhesion proteins, including ubiquitinated (UBB) focal adhesion (FA) proteins through NBR1, phosphorylated SRC (SRC p-Y416) through CBLC, and SRC-mediated phosphorylated PXN. (B) Autophagy negatively regulates Rho GTPases. Autophagy is activated by RHOA-ROCK signaling activity to target ARHGEF2 and RHOA for SQSTM1-dependent degradation through a negative feedback mechanism. Loss of autophagy can promote metastasis through increased RHOA activity. Autophagy and RAC negatively regulate one another, whereas CDC42 promotes autophagy. (C) Autophagy promotes anoikis-resistance. In detached cells and CTCs, autophagy is stimulated to suppress anoikis through several mechanisms, including EIF2AK3-ATF4-mediated increases in ATG gene expression, EIF2AK3-mediated suppression of MTORC1, and ROS-CCAR2-mediated IKK activation. (D) Autophagy suppresses EMT and fibrosis. EMT and fibrosis promote metastasis and exhibit mechanistic overlap. TGFB1 signals through SMAD, which promotes SNAIL- and TWIST-induced EMT and fibrosis. Autophagy negatively regulates EMT through SQSTM1-mediated degradation of SNAIL and by reducing SQSTM1-mediated stabilization of TWIST. Autophagy reduces FN1 and fibrosis by suppressing ROS to inhibit IL1B- and NFKB-induced fibrosis, and through MAP1S-dependent autophagic degradation of FN1.
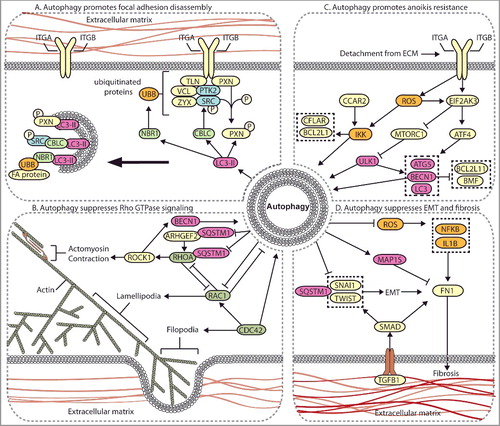
Figure 4. Autophagy contributes to integrin recycling and degradation. Cell migration is dependent on integrin recycling, where integrins are endocytosed, recycled back to the plasma membrane, or degraded in the lysosome. Canonical integrin recycling is depicted, including short-loop integrin recycling (pink arrows), long-loop integrin recycling (blue arrows), and endosome-mediated trafficking of integrins to the lysosome or to the plasma membrane for exosome secretion (black arrows). The autophagy pathway (green arrows) converges with and contributes to endosomal integrin trafficking pathways at several points, including in the formation of amphisomes, vesicle trafficking from RAB11 recycling endosomes to growing phagophores, and autophagy-mediated delivery of integrins to lysosomes.
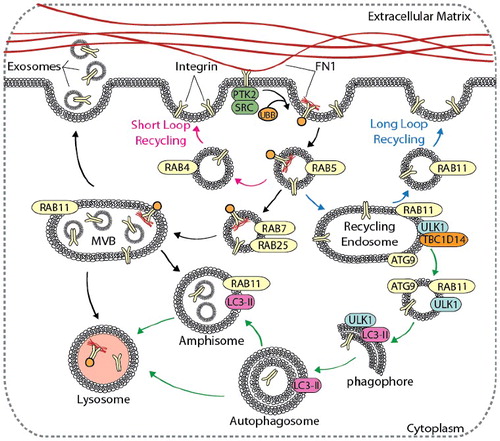
Figure 5. Autophagy compartmentalization promotes metabolic coupling between cancer cells and cancer-associated fibroblasts (CAFs). Autophagy-mediated metabolic coupling between cancer cells and stromal cells promotes tumor cell growth and metastasis. Loss of CAV1 promotes autophagy and catabolic metabolism through increased NFKB and HIF1A signaling, generating metabolic fuel, such as ketones and lactate, which are used to drive anabolism. Metabolite transport is mediated by increased expression of SLC16A4 and SLC16A1 on CAFs and cancer cells, respectively. Autophagy-mediated metabolic coupling suppresses apoptosis in cancer cells through increased TIGAR expression. CAV1 also promotes fibrosis by increasing SERPINE1 and FN1 expression.
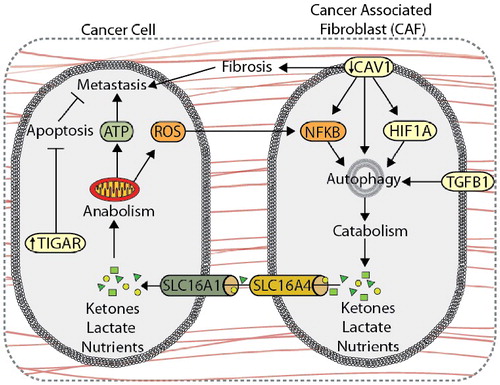
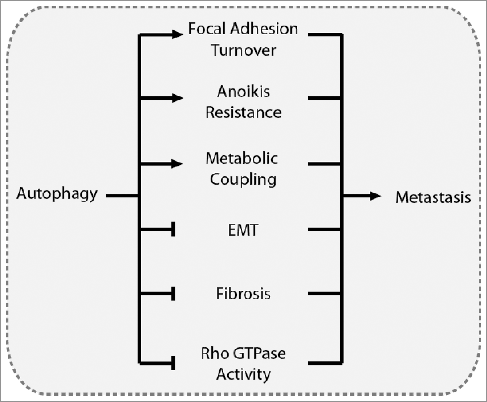
Figure 6. Overview of the driving mechanisms underlying the role of autophagy in metastasis. Autophagy can promote metastasis by contributing to focal adhesion turnover, anoikis resistance, and metabolic coupling with the tumor stroma. Conversely, autophagy can suppress metastasis by inhibiting EMT, tumor fibrosis, and Rho GTPase activity.