Figures & data
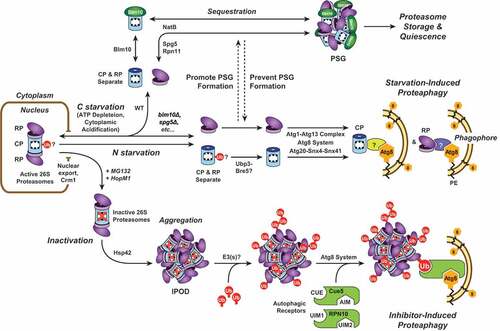
Figure 1. A model for proteasome storage versus proteasome degradation upon nutrient starvation. When cells are subjected to nitrogen or carbon starvation, proteasomes are exported from the nucleus, and the CP and RP separate. Upon nitrogen starvation, both CP and RP are rapidly encapsulated by expanding phagophores and delivered to the vacuole for degradation. Deubiquitination of one or more CP subunits by Ubp3 might be required; whether specific Atg8-binding autophagy receptors are involved remains unknown. In contrast, carbon starvation triggers CP and RP aggregation into PSGs. This requires numerous factors, including Blm10 for the CP, Spg5 and Rpn11 for the RP, and the NatB N-terminal acetylation complex for both. Preventing sequestration of proteasomes into PSGs leads to their Atg1- and Atg8-dependent degradation. An additional proteaphagic route is stimulated by proteasome inhibition. Here, proteasomes exported from the nucleus aggregate in an Hsp42-dependent manner into IPOD-like structures that are distinct from PSGs. The aggregated proteasomes are then ubiquitinated (Ub) by one or more E3 ligase(s), facilitating recognition by the selective proteaphagy receptors Cue5 or RPN10. By simultaneous interactions with Atg8/ATG8, these receptors deliver inactive proteasomes to enveloping autophagic vesicles for final degradation in the vacuole.