Figures & data
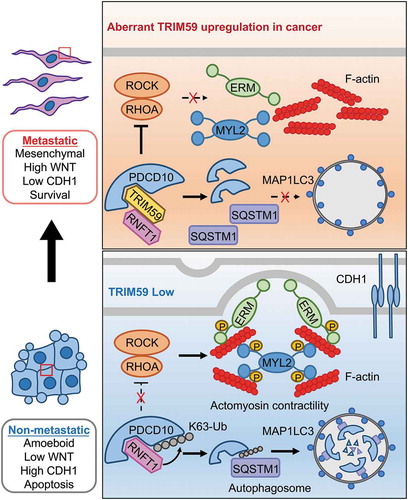
Figure 1. A working model depicting the TRIM59-PDCD10 interplay during cancer metastasis. TRIM59 is essential for breast cancer cell mesenchymal movement and cell survival by maintaining low cell adhesion and high WNT signaling. TRIM59 stabilizes PDCD10 by inhibiting RNFT1-induced K63 ubiquitination and subsequent SQSTM1-selective autophagic degradation. TRIM59 ablation removes the stabilizing effect on PDCD10 to cause autophagic degradation of PDCD10, and further activates the downstream RHOA-ROCK signaling to promote the phosphorylation of MYL2/MLC2 and ERM. These changes contribute to the amoeboid phenotype and focal adhesion formation, ultimately curtailing tumor formation and metastasis.