Figures & data
Figure 1. Dictyostelium mutants lacking γ-secretase component orthologs are unable to endocytose at wild type levels. Dictyostelium cells were shaken in media containing fluorescent TRITC-Dextran, and fluorescence uptake was used to monitor macropinocytosis over time, in wild-type cells, PsenA−/B−, Aph-1− and Ncstn− cells, and PsenA−/B− following rescue by the proteolytic and non-proteolytic human PSEN1 proteins (PsenA−/B−::PSEN1-GFP and PsenA−/B−::PSEN1DD). (a) Representative images showing uptake of TRITC-Dextran by wild-type cells over a 60-min period. Scale bar: 10μ m. (b) Quantification of macropinocytosis over 60 min (±SEM). (c) Rate of macropinocytosis over 60 min (±SD). Data are provided from at least three independent experiments with technical duplicates. ++p > 0.01 to wild type, **p > 0.01 to PsenA−/B−.
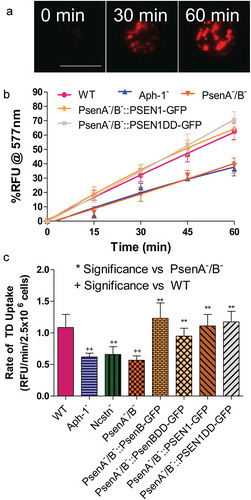
Figure 2. Dictyostelium mutants lacking γ-secretase component orthologs are unable to development under varying pH conditions. The development of Dictyostelium, through starvation over a 24-h period on nitrocellulose filters, leads to the formation of fruiting bodies consisting of round spore heads held aloft by a stalk, and provides a qualitative approach to monitor development in mutants. (a) At pH 7 and pH 9, wild type cells are capable of normal multi-cellular development forming fruiting bodies. In contrast, PsenA−/B− cells are unable to develop at pH 7, forming short variable structures, and development is further inhibited at pH 9 where cells are unable to aggregate. (b) Aph-1− and Ncstn− cells form wild-type fruiting bodies at pH 7 but development is blocked at pH 9 (see also Figure S4). (c) Under acidic conditions, pH 5, wild-type cells form morphologically normal fruiting bodies, and PsenA−/B− development is partially rescued, showing the formation of small fruiting bodies with round spore heads and stalks, similar to that shown for wild-type cells. Images are representative of triplicate experiments. Scale bar: 1 mm.
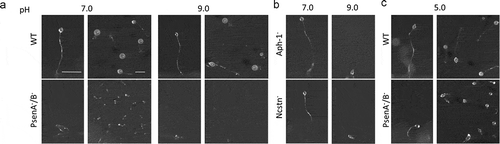
Figure 3. Dictyostelium mutants lacking γ-secretase component orthologs show abnormal lysosomal activity. Phagosome degradation is quantified by monitoring the increase in fluorescence of DQ-BSA-coated beads, taken up by phagocytosis, which becomes unquenched upon hydrolysis. This approach was used to assess in wild type cells, PsenA−/B−, and Aph-1− cells, and PsenA−/B− cells following rescue by the proteolytic and non-proteolytic Dictyostelium psenB or the equivalent human PSEN1 proteins (PsenB-GFP and PsenBDD-GFP or PSEN1-GFP and or PSEN1DD-GFP) respectively. (a) Quantification of proteolysis shows loss of a functional γ-secretase complex reduces phagolysosomal degradation, and this is restored by proteolytically active or inactive human PSEN1, from quadruplicate independent experiments (±SEM), which is reflected in (b) the rate of lysosomal acidification in (±SD). +P < 0.05. (c) Dictyostelium cells exhibit puncta of GFP-Atg8 that may be tracked (arrow) over time to determine the quenching time of GFP due to acidification. (d) Quantification of GFP-Atg8 quenching time shows an increase in the absence of a functional γ-secretase complex, and this is restored by both Dictyostelium and human presenilin proteins (both proteolytically active and inactive) (n = 25). +++p > 0.001 to wild type, ***p > 0.001 to PsenA−/B.
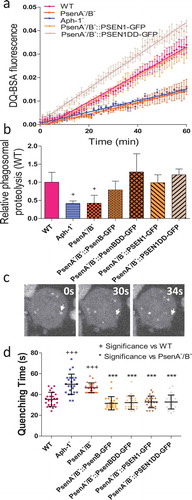
Figure 4. Dictyostelium mutants lacking γ-secretase component orthologs show aberrant size and localisation of GFP-Atg8. Visualization of GFP-Atg8 localisation in wild type cells, PsenA−/B− and Aph-1− and PsenA−/B− cells following rescue by the proteolytic and non-proteolytic Dictyostelium psenB or the equivalent human PSEN1 proteins (PsenB-GFP and PsenBDD-GFP or PSEN1-GFP and or PSEN1DD-GFP) respectively. (a) Wild-type cells show multiple small and distributed GFP-Atg8-containing autophagosomes, whereas a single large punctum is seen in a proportion of PsenA−/B− and Aph-1− cells, that is no longer observed when cells are rescued following expression of Dictyostelium psenB orhuman PSEN1 (proteolytically active or inactive). Scale bar: 10μ m. (b) Quantification of the size of GFP-Atg8 -containing autophagosomes shows an increase in the absence of a functional γ-secretase complex, and this is restored by both Dictyostelium and human presenilin proteins (both proteolytically active and inactive). Data are derived from triplicate experiments measuring approximately 50 cells per experiment. ‘+’ compares to Aph-1−, ‘*’compares to PsenA−/B−, where * or + is P < 0.05, ** or ++ is P < 0.01, *** or +++ is P < 0.05.
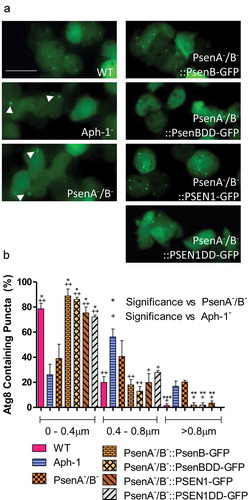
Figure 5. Dictyostelium mutants lacking γ-secretase component orthologs show decreased autophagic flux. Representative western blot analysis using an anti-GFP antibody enables comparison of levels of free GFP to GFP-Atg8 in the presence or absence of protease inhibitor (PI) treatment, allowing the comparison of wild type, Aph-1−, and PsenA−/B− cell autophagic flux, and following rescue by the proteolytically active human PSEN1 protein (PSEN1-GFP). (a) Levels of free GFP are reduced in wild-type cells following PI treatment. In both Aph-1− and PsenA−/B− cells, free GFP levels are reduced in the absence of PI, and remain low following PI treatment. Expression of PSEN1-GFP in the PsenA−/B− cells restores free GFP levels in untreated cells, and PI sensitivity. Endogenously biotinylated mitochondrial protein MCCC1 was used as a loading control. (b) Quantitation of free GFP to GFP-Atg8 ratios shows the absence of a functional γ-secretase complex reduces autophagic flux, and this is restored by the human PSEN1 protein. Data are derived from triplicate independent experiments (±SEM). ‘*’ compares to wild type, ‘+’ compares to PsenA−/B−, where * or + is P < 0.05, ** or ++ is P < 0.01; NS, not significant.
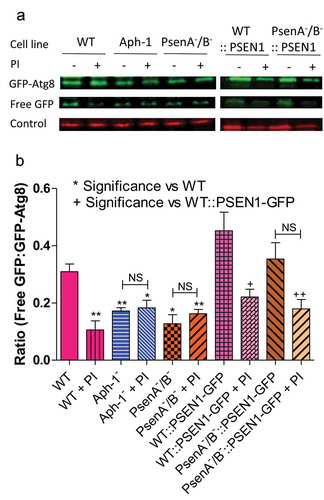
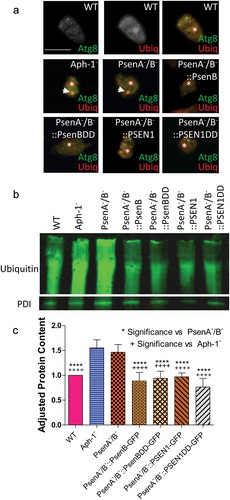
Figure 6. Dictyostelium mutants lacking γ-secretase component orthologs show ubiquitination defects. Analysis of ubiquitination levels in wild-type cells, PsenA−/B− and Aph-1− and PsenA−/B− cells following rescue by the proteolytic and non-proteolytic Dictyostelium PsenB or the equivalent human PSEN1 proteins (PsenB-GFP and PsenBDD-GFP or PSEN1-GFP and PSEN1DD-GFP) respectively. (a) In cells expressing GFP-Atg8, immunofluorescence analysis shows co-localization of GFP and ubiquitin in single large puncta in Aph-1− and PsenA−/B− cells that are absent in wild-type cells, and are absent following rescue with the presenilin proteins. Scale bar: 10μ m. (b) Representative western blot analysis using anti-ubiquitin in the wild type, mutants and following rescue as indicated. Anti-PDI was used as a loading control for normalisation. (c) Quantification of anti-ubiquitin western blot shows a ~ 50% increase in large molecular weight ubiquitinated protein in cells lacking a functional γ-secretase complex when compared to normalized wild type levels. This increase is restored to wild type levels in PsenA−/B− cells expressing Dictyostelium PsenB or human PSEN1 proteins regardless of proteolytic activity. Data are derived from 5 independent experiments.