Figures & data
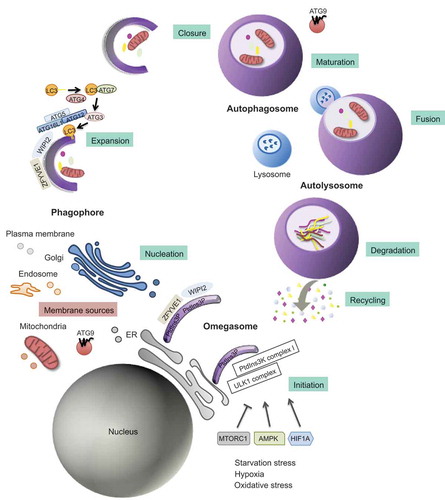
Figure 1. Schematic representation of the autophagy process. Autophagy is a multi-stage process that comprises several steps: initiation, expansion, closure, and fusion with the endolysosomal system. The process initiates with the progressive sequestration of a portion of the cytoplasm that is enclosed within a double-membrane (a phagophore) to finally form an autophagosome. The initiation involves the transmission of the autophagy signal, which is triggered by multiple conditions, such as nutrient starvation, and canalized through specific cell sensors such as AMP-activated protein kinase (AMPK), HIF1A (hypoxia-inducible factor alpha) and mechanistic target of rapamycin kinase complex 1 (MTORC1) to the membrane of origin at which the nucleation takes place. As a consequence, there is a recruitment of the key initiation complexes. The ULK1 (unc-51 like autophagy activating kinase 1) complex plays a central role in the initiation stage, and it is the target of these signaling pathways and, when activated, triggers the nucleation of the phagophore by phosphorylating components of the class III phosphatidylinositol 3-kinase (PtdIns3k) complex I. Then, WIPI2 (WD repeat domain, phosphoinositide interacting 2), and ZFYVE1 (zinc finger FYVE domain containing 1) are recruited to the omegasome. WIPI2 binds and is activated by phosphatidylinositol 3-phosphate (PtdIns3P). Once activated, WIPI2 recruits at phagophore assembly sites the ATG12–ATG5-ATG16L1 complex that enhances the ATG3-mediated conjugation of LC3 (microtubule-associated protein light chain 3) to phosphatidylethanolamine. LC3-I is converted into its lipidated form, LC3-II. Membrane material for the nucleation of the phagophore and the elongation of the autophagic membrane arises from the ER, mitochondria, recycling endosomes, Golgi apparatus, and plasma membrane from which ATG9-containing vesicles provide portions of lipid bilayers. Finally, the expansion continues until the complete formation and closure of the autophagosome. The outer membrane of the autophagosome subsequently fuses with the lysosome, giving rise to the autolysosome. After lysosomal digestion, the sequestered cytoplasmic material is degraded into amino acids and macromolecules, transported across the lysosomal membrane to the cytosol, and finally recycled for anabolic processes
Box 1. Definitions
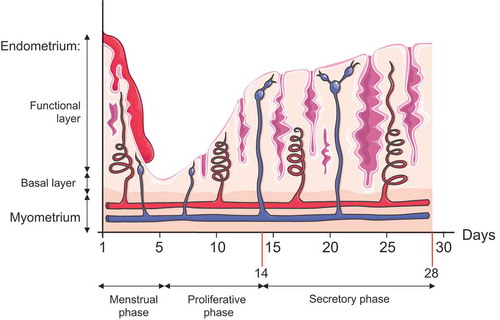
Figure 2. Illustration of endometrial layers and tissue components dynamic changes during the course of the different phases of the menstrual cycle. The endometrium, composed of a single layer of columnar epithelial cells plus stroma, can be subdivided into two layers: the functional, which faces the uterine cavity, and the basal layer in direct contact with the myometrium. Both layers undergo structural changes during the menstrual cycle. Menstruation constitutes the first phase of the menstrual cycle, and it is characterized by the complete desquamation of the functional layer of the endometrium and bleeding (menses). This stage precedes the proliferative phase, characterized by the increase in the synthesis and secretion of estrogens as the ovarian follicles mature (ovarian follicular phase). The estrogens will promote the regeneration of the functional layer from the basal layer of the endometrium. By day 14, a peak in LH secretion triggers a decrease in estrogen production, ovulation, and the formation of the corpus luteum (luteal ovarian phase), which represents the primary source of progesterone. Progesterone will prepare the endometrium for future implantation by fostering global endometrial thickening characterized by increased spiral artery length and coiling, uterine secretions, and reduced smooth muscle cell contractility. In the absence of pregnancy, the demise of the corpus luteum will cause a dramatic drop in the progesterone and estrogen levels, and the initiation of the menstruation starts again
Box 2. Definitions
Table 1. Targeting autophagy in endometrial cancer
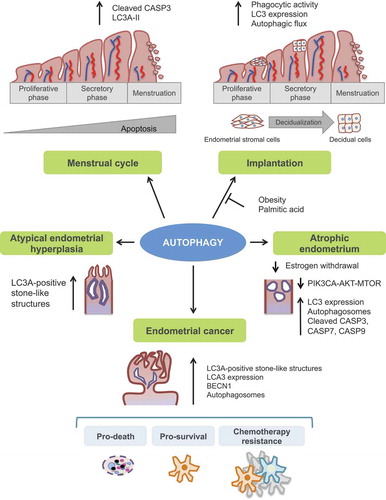
Figure 3. Illustration of the principal autophagy-related function into the human endometrium. Autophagy has a crucial role in both normal and pathological endometrium and has been linked to endometrial cancer development