Figures & data
Figure 1. Pro-inflammatory cytokine induction, autophagic flux impairment and NFKB activation by TcdB in human PMA-differentiated THP-1 macrophages. (A) Effects of exposure to TcdB (10 ng/ml) for the indicated time periods on the mRNA levels of IL1B, IL8 and CXCL2 in cultured THP-1 macrophages. (B) Effects of exposure to TcdB at the indicated concentrations for 6 h on the mRNA levels of IL1B, IL8, and CXCL2 in cultured THP-1 macrophages. LPS (100 ng/ml) was used as a positive control. (C) Exposing macrophages to TcdB (10 ng/ml) for 6 h decreased the protein levels of NFKBIA/IKB, enhanced NFKB RELA/p65 phosphorylation at serine 536, and promoted the nuclear translocation of NFKB RELA/p65 (green). Cytoplasmic and nuclear localization of NFKB RELA/p65 were visualized by immunofluorescence. Nuclei (blue) were labeled with DAPI. A total of 50 cells from each group were randomly selected for quantification by the ImageJ software. Scale bar: 5 µm (D) Exposing macrophages to TcdB (10 ng/ml) for 6 h led to increased protein levels of both LC3-II and SQSTM1/p62 (left panel). Exposing macrophages to TcdB (10 ng/ml) in the presence of bafilomycin A1 (200 μM) for 6 h induced no further increase in SQSTM1/p62 protein levels (right panel). (E-F) Knockdown of SQSTM1 with siRNA abolished TcdB (10 ng/ml; 6 h)-induced (E) NFKB RELA/p65 phosphorylation at serine 536 and (F) mRNA expression of IL1B, IL8, and CXCL2. Protein and mRNA levels were quantified by Western blots and RT-qPCR, respectively. Results are expressed as mean ± S.E.M. from three independent experiments.
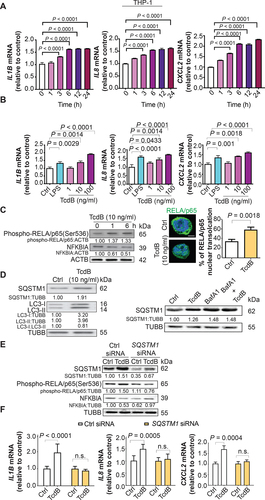
Figure 2. Impairment of autophagic flux and lysosome acidification by TcdB in macrophages. (A) Ultrastructural alterations induced by TcdB (10 ng/ml, 6 h) in PMA-differentiated THP-1 cells were visualized by TEM. The typical images of autolysosomes (arrow) and autophagosomes enclosing intact undigested cargo (arrowhead) are shown at higher magnification. Scale bar: 2 µm (Enlarged; Scale bar: 200 nm) (n = 3 for each treatment; 10 fields for each biological replicate were used for quantification). (B) Autophagosome-lysosome fusion was assessed by measuring the colocalization of LC3-positive autophagosomes with LAMP1-positive lysosomes in PMA-differentiated THP-1 cells. TcdB (10 ng/ml, 6 h) induced autophagosome-lysosome fusion to an extent similar to that of rapamycin (500 nM, 6 h), a drug for inducing autophagy with unimpeded flux. Scale bar: 5 µm (C) TcdB (10 ng/ml, 6 h) reduced the intensity of LysoTracker Red without affecting total lysosome number as stained by immunofluorescence for LAMP1. A total of 50 cells from each group were randomly selected for quantification by the ImageJ software. Scale bar: 5 µm (D) TcdB-induced impairment of lysosome/autolysosome acidification in PMA-differentiated THP-1 cells was confirmed by mCherry-GFP-LC3 assay. Acidified (digestive) and non-acidified (non-digestive) LC3-positive autophagosomes were visualized under a confocal microscope. Bafilomycin A1 (BafA1; 200 μM, 6 h) was used as a control to impair lysosome acidification and autophagic flux. A total of 20 cells from each group were randomly selected for quantification by the ImageJ software. Scale bar: 5 µm (E) Effects of TcdB (6 h) at the indicated concentrations on the proteolytic cleavage of pro-CTSD into the mature form in PMA-differentiated THP-1 cells. LPS did not affect the proteolytic cleavage. Scale bar: 5 µm (F) TcdB (6 h) at the indicated concentrations reduced the activities of two classical lysosomal enzymes – ACP2 and NAGLU in human and murine macrophages. Results are expressed as mean ± S.E.M. from three independent experiments.
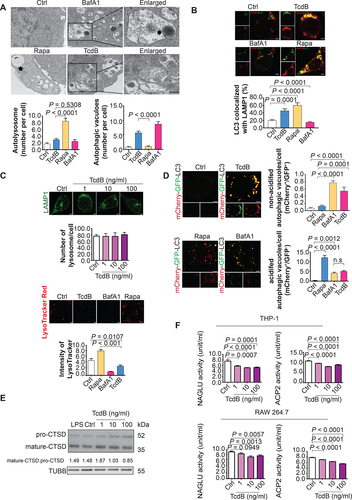
Figure 3. TcdB-mediated pro-inflammatory response through the CTNNB1/β-catenin-LEF-MITF-lysosome proton pump pathway in macrophages. (A) Effects of TcdB (10 ng/ml; 6 h) on mRNA levels of three MITF/TFE family transcriptional factors (MITF, TFEB, TFE3) are shown. The downregulation of MITF by TcdB at protein level was confirmed. (B) TcdB (10 ng/ml; 6 h) reduced mRNA levels of the lysosomal proton pump subunits (ATP6V0C, ATP6V1H, ATP6V0B, ATP6V0E1). (C) ChIP-sequencing analysis showed that TcdB diminished the occupancy of MITF at regions (−2000 bp to +2000 bp) near the transcription start site (TSS) of the pulled down protein-coding genes in PMA-differentiated THP-1 macrophages. The enrichment of the pulled down genes in biological processes by KEGG analysis is shown. Besides, TcdB diminished the occupancy of MITF near TSS of genes encoding lysosomal proton pump subunits (ATP6V0C, ATP6V1H, ATP6V0B, ATP6V0E1). TYR and G6PD serve as positive and negative control for MITF occupancy, respectively. (D) Effects of TcdB (10 ng/ml; 6 h) on the protein levels of WNT signaling transcription factors (TCF7L2, LEF1 and active CTNNB1/β-catenin) and nuclear translocation of CTNNB1/β-catenin (green) are shown. Cytoplasmic and nuclear localization of CTNNB1/β-catenin were visualized by immunofluorescence. Nuclei (blue) were labeled with DAPI. A total of 50 cells from each group were randomly selected for quantification by the ImageJ software. Scale bar: 20 µm. (E) TcdB reduced the promoter activity of MITF as determined by luciferase assay in PMA-differentiated THP-1 cells. The effects of site-directed mutagenesis of LEF1-binding site on MITF promoter activity in the absence or presence of TcdB (10 ng/ml; 6 h) are shown. (F) Effects of TcdB without or with enforced expression of MITF isoforms (MITF-A, MITF-M, MITF-D) on autophagic flux and proteolytic CTSD processing in PMA-differentiated THP-1 cells are shown. Cells transfected with EGFP-encoding plasmids were used as a control. (G and H) Effects of TcdB without or with enforced expression of MITF isoforms on mRNA levels of (G) lysosomal proton pump subunits and (H) pro-inflammatory cytokines in PMA-differentiated THP-1 cells are shown. Protein and mRNA levels were quantified by Western blots and RT-qPCR, respectively. Results are expressed as mean ± S.E.M. from three independent experiments. *, p < 0.05; **, p < 0.01; ****, p < 0.0001 when compared with the TcdB-unexposed, EGFP-transfected group. ##, p < 0.01; ####, p < 0.0001 when compared with the TcdB-exposed, EGFP-transfected group.
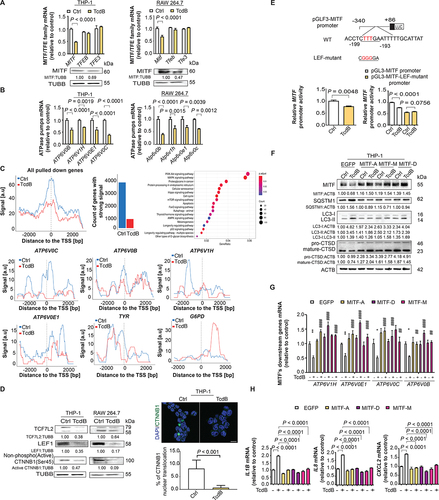
Figure 4. Correlation of MITF mRNA expression and autophagic flux impairment with TcdB released from clinical isolates of C. difficile. PMA-differentiated THP-1 cells were exposed to the medium of stationary-phase culture of different C. difficile clinical isolates (all are ribotype 002) for 6 h. TcdB concentrations were measured by ELISA. (A) Correlation between MITF mRNA levels and TcdB concentrations was shown. (B) Correlations between protein levels of autophagic flux markers (LC3-II and SQSTM1/p62; after normalization with ACTB) and TcdB concentrations are shown. Correlation coefficients and P values were derived from the Pearson correlation test.
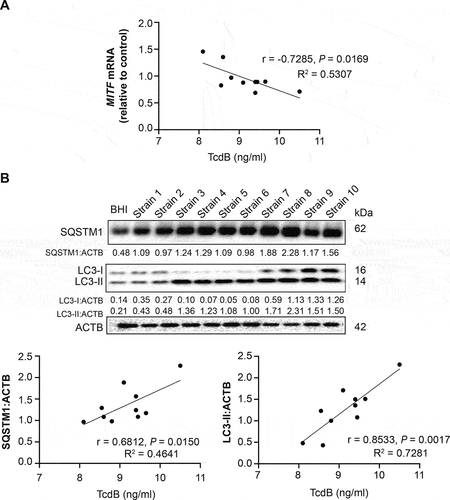
Figure 5. Enforced expression of MITF abolished TcdB-impaired phagocytosis. TcdB (10 ng/ml; 6 h) attenuated the (A) cell viability and (B-C) phagocytic activity of THP-1 (PMA-differentiated) and RAW 264.7 macrophages as evidenced by (B) E. coli internalization assay and (C) FITC-dextran uptake assay. For the E. coli internalization assay, THP-1 and RAW 264.7 were pre-treated with TcdB and then incubated with E. coli at 100 MOI for 4 h. Adhered extracellular bacteria were subsequently killed by gentamicin (100 μg/ml). The internalized bacteria were examined by LB agar plating after 24 h. The results of E. coli internalization assay were normalized with cell viability. For the FITC-dextran uptake assay, THP-1 and RAW 264.7 macrophages were incubated for 6 h with TcdB and exposed for 1 h to FITC-dextran (250 μg/ml). The fluorescence signals were then analyzed by flow cytometry. (D) THP-1 cells transfected with different isoforms of MITF abolished TcdB-mediated inhibition of phagocytosis of E. coli. Results are expressed as mean ± S.E.M. from three independent experiments.
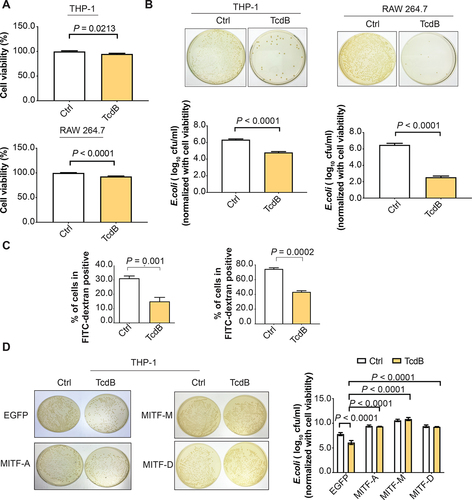
Figure 6. Effects of lysosome activators 1α,25-dihydroxyvitamin D3 and carbamazepine on TcdB-induced autophagic flux impairment and cytokine response in human PMA-differentiated THP-1 macrophages. Effects of pre-treatment with 1α,25-dihydroxyvitamin D3 (1,25-VD3) and carbamazepine (CBZ) at the indicated concentrations for 24 h on TcdB (10 ng/ml; 6 h)-induced (A) impairment of autophagic flux and proteolytic cleavage of CTSD; (B) downregulation of mRNA levels of MITF and lysosomal proton pump subunits; (C) mRNA expression of pro-inflammatory cytokines in human macrophages are shown. Protein and mRNA levels were quantified by Western blots and RT-qPCR, respectively. (D) Protein levels of pro-inflammatory cytokines in human macrophages were measured by ELISA. Results are expressed as mean ± S.E.M. of 3 independent experiments. ****, p < 0.0001 when compared with TcdB-unexposed vehicle control. ####, p < 0.0001 when compared with TcdB-exposed vehicle control.
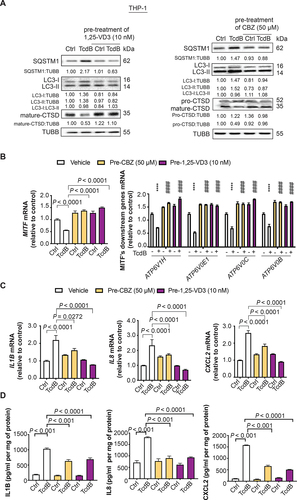
Figure 7. Amelioration of symptoms and histological damage by lysosome activators vitamin D3 and carbamazepine in a murine CDI model. (A) Mice were given cefoperazone in drinking water (0.5 mg/ml) for 10 days before being challenged with 1 × 108 colony-forming units of a TcdB-hyperproducing C. difficile strain (VPI10463) to establish the CDI model. All mice were sacrificed 2 days after CDI. Effects of pre-treatment with (B – E) vitamin D3 (2300 IU/kg by oral gavage) or (F – I) carbamazepine (CBZ; 50 mg/kg by oral gavage) every other day for 2 weeks before C. difficile challenge on (B and F) H&E sections of mice colonic mucosa, (C and G) weight loss, (D and H) severity of diarrhea, and (E and I) histopathological scores of colon tissues 2 days post-infection are shown. Scale bar: 200 µm **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 significantly different between indicated groups (pooled from three batches of experiments; BHI group, n = 10; C. difficile group, n = 10; CBZ group, n = 10; C. difficile with CBZ group, n = 10; Vitamin D3 group, n = 5; C. difficile with vitamin D3 group, n = 13).
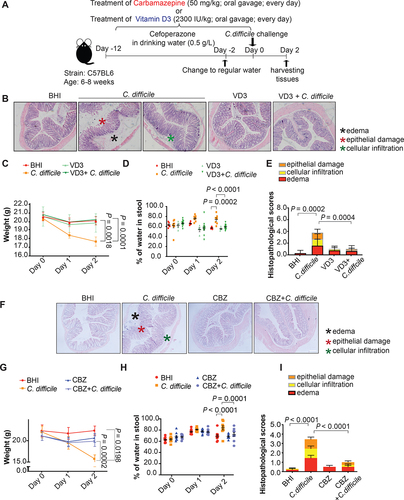
Figure 8. Attenuation of Mitf downregulation, lysosome dysfunction, and pro-inflammatory cytokine expression in colonic macrophages by vitamin D3 and carbamazepine in the murine CDI model. (A) Workflow of isolation of colonic macrophages is shown. (B-C) Effects of CDI without or with pre-treatment of (B) vitamin D3 (VD3; 2300 IU/kg by oral gavage) and (C) carbamazepine (CBZ; 50 mg/kg by oral gavage) every other day for 2 weeks on mRNA expression of MITF and the four lysosomal proton pump subunits in isolated colonic macrophages are shown (pooled from three batches of experiments; BHI group, n = 9; C. difficile group, n = 9; CBZ or VD3 group, n = 6 each; C. difficile with CBZ or VD3 group, n = 9 each). (D) Effects of CDI without or with pre-treatment of vitamin D3 or carbamazepine on mean fluorescence intensity (MFI) of LysoTracker Red staining in isolated colonic macrophages are shown. (E-F) Effects of CDI without or with pre-treatment of (E) vitamin D3 and (F) carbamazepine on mRNA expression of Il1b, Il8 and Cxcl2 in isolated colonic macrophages are shown.
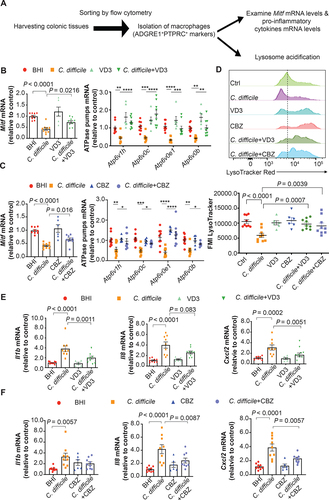
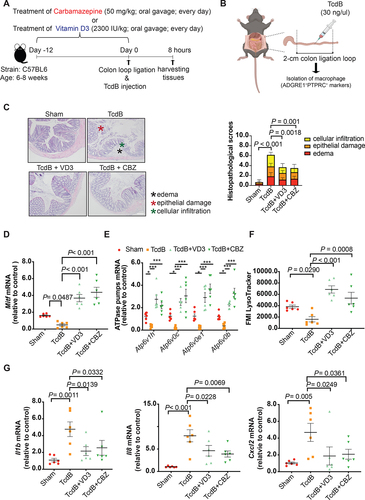
Figure 9. Attenuation of Mitf downregulation, lysosome dysfunction, and pro-inflammatory cytokine expression in colonic macrophages by vitamin D3 and carbamazepine in the murine colon loop ligation model. (A) Workflow of pre-treatment with lysosome activators in the colon loop ligation model is shown. (B) A schematic illustration of the colon loop ligation model. (C) Histopathological scores of sealed colon segment tissues. Scale bar: 200 µm (D-E) mRNA expression of Mitf and the four lysosomal proton pump subunits in isolated colonic macrophages are shown. (F) Effects of TcdB without or with pre-treatment of vitamin D3 (VD3) or carbamazepine (CBZ) on mean fluorescence intensity (MFI) of LysoTracker Red staining in isolated colonic macrophages are shown. (G) Effects of TcdB without or with pre-treatment with vitamin D3 or carbamazepine on mRNA expression of Il1b, Il8 and Cxcl2 in isolated colonic macrophages are shown. **, p < 0.01; ***, p < 0.001 significantly different between indicated groups (pooled from three batches of experiments; Sham group, n = 6; TcdB group, n = 6; TcdB with CBZ or VD3 group, n = 6 each).