Figures & data
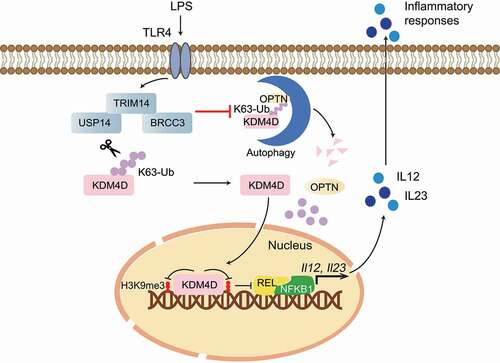
Figure 1. A working model to elucidate that OPTN-mediated selective autophagic degradation of KDM4D epigenetically regulates inflammation. The TRIM14-USP14-BRCC3 complex reduces the K63-linked ubiquitin chains of KDM4D to inhibit OPTN-mediated autophagic degradation of KDM4D, which is responsible for removing the H3K9me3 modification at Il12 and Il23 promoters to promote the expression of proinflammatory cytokines IL12 and IL23 to enhance inflammation. LPS, lipopolysaccharide.