Figures & data
Figure 1. Autophagy and its mechanism in COVID-19. The autophagy process can be divided into four stages: (1) initiation, (2) nucleation, (3) expansion, and (4) maturation/degradation. In the initial stage, bioenergy stress in the host cell increases after SARS-CoV-2 invasion, thus increasing the level of cytoplasmic AMP: ATP, leading to activation of AMPK and phosphorylation/deactivation of MTORC1. Then in the nucleation stage, the ULK1 complex is activated, thus activating the PtdIns3K complex and producing a PtdIns3P-rich area (PtdIns3P) on the surface of omegasomes. PtdIns3P then recruits the WIPI and ZFYVE1 proteins that bind to the omegasome. In the expansion stage, ATG7 and ATG3 synergistically prime MAP1LC3/LC3, WIPI proteins recruit conjugation factors, including the ATG16L1 complex, leading to the conjugation of MAP1LC3/LC3 to phosphatidylethanolamine (generating LC3-II) to form APs. The last stage is maturation/decomposition, APs and lysosomes fuse to form autolysosomes. Substances wrapped in APs are degraded.
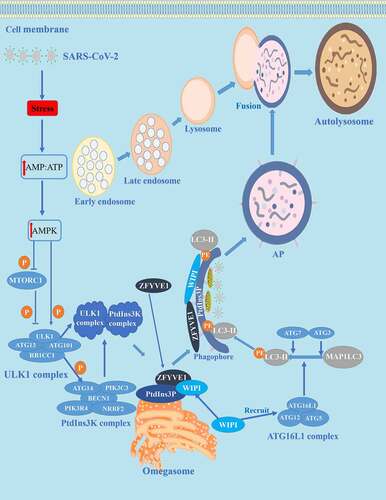
Figure 2. NETosis and its mechanism in COVID-19. NETosis is a process of death with release of NET accompanied by auto-inflammatory factors and antigens. Calcium ionophore activates the CRAC channel of the cytoplasmic membrane and mobilizes Ca2+ in the endoplasmic reticulum, increasing the concentration of Ca2+ in the cytoplasm. Subsequently, Ca2+ overload and oxidative stress in the mitochondrial matrix lead to nonselective mitochondrial pores and the formation of MTROS. The release of MTROS into the cytoplasm activates PRKC and NADPH oxidase and induces the formation of NETs independently of MTROS and MPTP. Under stress conditions such as invasion of SARS-CoV-2, the NADPH oxidase complex is activated, subsequently producing large amounts of ROS. The azurosome is a protein complex that contains the ELANE and MPO proteins. Under ROS, the azurosome releases ELANE and MPO into the cytoplasm. In addition, the azurosomes dissociate in the presence of ROS, causing ELANE and MPO to enter the nucleus to decompose laminin and histones. PADI4 citrullinates histones, resulting in nucleocapsid agglomeration and chromatin depolymerization. DNA fibers or nuclear chromatin, released into the cytoplasm, combined with cytoplasmic proteins, granular proteins, histones, and F3, encapsulate the cytoplasm and finally in the extracellular form of NETs. cDNA and histone promote the release of pro-inflammatory cytokines and TCC. Neutrophils also release ELANE and MPO, which dissolve invasive pathogens such as SARS-CoV-2, digest particles, and induce tissue and bystander damage.
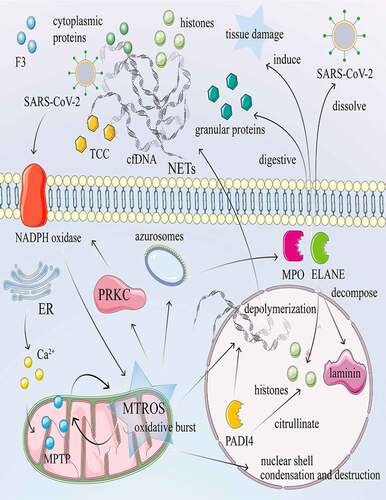
Table 1. Potential therapeutic targets of autophagy in COVID-19.
Table 2. Potential therapeutic targets for NETosis in COVID-19.
