Figures & data
Figure 1. Dynamics of enzymatic 5mC oxidation during hepatic differentiation. (A) Co-detection of 5caC with 5hmC and DAPI (upper row) or of the indicated differentiation markers with DAPI (middle row and lower row for HEP ENDO 96 h stage) in undifferentiated REBL-PAT hiPSCs and at specified stages of their differentiation toward hepatic endoderm. Cell cultures were immunostained in parallel under the same experimental conditions and imaged at identical settings. UNDIFF – undifferentiated cells; ENDO 24 h and 72 h – cells 24 and 72 h after definitive endoderm induction; FOREGUT 24 h and 72 h – cells 24 and 72 h after induction of foregut endoderm; HEP ENDO 24 h and 96 h – cells 24 and 96 h after induction of hepatic endoderm. Merged views and individual channel for DAPI are shown. Scale bars are 15 µm. (B) Quantification of 5 hmC and 5caC signal intensities in REBL-PAT hiPSCs at the specified stages of their differentiation into hepatocytes. Experimental error is shown as SD ***P < 0.001; **P<0.01. (C) DNA dot blot of 5caC and 5mC in undifferentiated hiPSCs and in differentiating cells 24 h after induction of hepatic endoderm. The amounts of DNA loaded on to membranes are indicated. (D) dmC/dC and dhmC/dmC ratios obtained from the quantification of MS peaks in undifferentiated hiPSCs and at indicated stages of their differentiation toward foregut and hepatic endoderm. Experimental error is shown as SD.
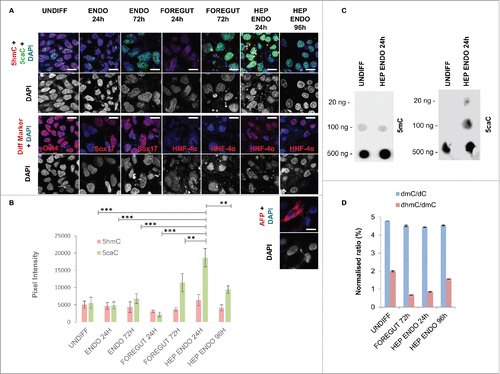
Figure 2. 5caC accumulates at promoter regions of hepatocyte markers at the onset of their expression. (A) Relative expression of TET1/2/3 and TDG mRNAs at the specified stages of hepatic differentiation. In addition to the stages of differentiation presented in stages of hepatocytes maturation (MATURATION) are also shown. (B) Relative expression (Fold change, FC) of the specified hepatocyte markers at the indicated stages of hepatic differentiation. (C) 5caC DIP of indicated promoters in cells at specified stages of hepatic differentiation. TBx3 and HNF-4α DIP results are shown using log scale. Experimental error is presented as SD.
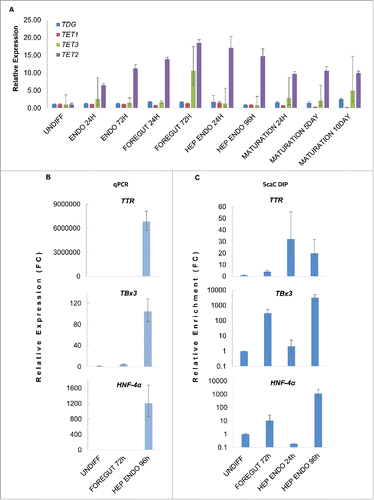
Figure 3. Nuclear distribution of 5hmC and 5caC during specification of foregut endoderm and during hepatic endoderm commitment. (A) Distribution of 5caC, 5hmC, and DAPI signals in the nuclei of representative cells at indicated stages of differentiation after induction of foregut (FOREGUT 24 h, FOREGUT 72 h) and hepatic endoderm (HEP ENDO 24 h). Merged views together with corresponding 2.5XD signal intensity plots and 2.5XD signal intensity plots for individual channels are presented. (B) 5hmC, 5caC and DAPI signals in 2 cells exhibiting 5caC staining of different intensities 96 h after induction of hepatic endoderm. Merged view and individual channels are shown. (C) 5caC/5hmC FI (fluorescence intensity) colocalization plots for representative images of the nuclei of FOREGUT 72 h and HEP ENDO 24 h cells depicted in (A). (D) Boxplot showing 5caC:5hmC colocalization coefficient values for cell populations at the indicated differentiation stages. Twenty to sixty individual cells were analyzed for each stage. ***P < 0.0001, ns – not statistically significant.
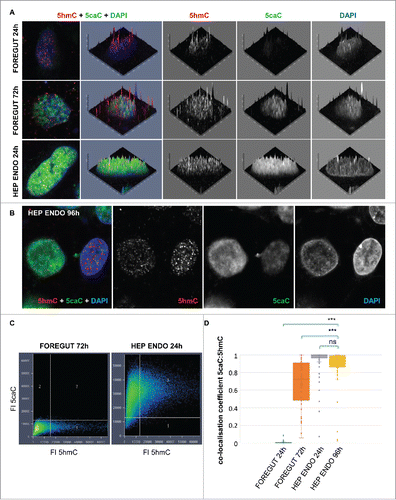
Figure 4. 5caC staining intensity does not correlate with the levels of HNF-4α expression during hepatic endoderm commitment. (A) Co-detection of 5caC with HNF-4α and DAPI in the culture of differentiating cells 96 h after induction of hepatic endoderm. Merged view is shown. Individual nuclei with different levels of 5caC and HNF-4α staining presented in (B-E) are marked. (B-E) Distribution of 5caC, HNF-4α, and DAPI staining in 4 individual nuclei exhibiting different intensities of 5caC and HNF-4α signals. Merged views together with corresponding 2.5XD signal intensity plots and 2.5XD signal intensity plots for individual channels are presented.
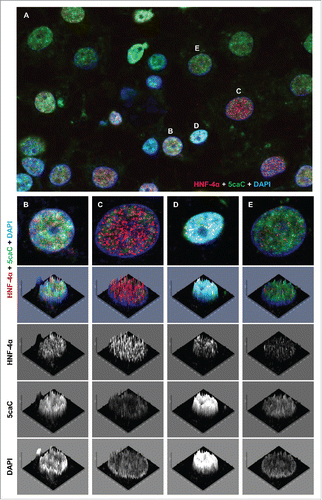
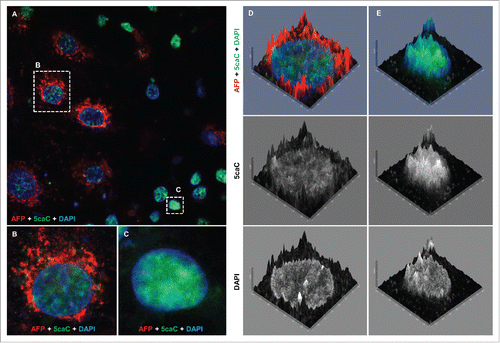
Figure 5. The levels of 5caC staining drop concurrently with the onset of AFP expression during hepatic endoderm commitment. (A) Co-detection of 5caC with AFP and DAPI in the culture of differentiating cells 96 h after induction of hepatic endoderm. Merged view is shown. Individual cells with different levels of 5caC and AFP staining presented in (B) and (C) are marked with dotted rectangles. (B-C) Co-detection of 5caC with AFP and DAPI in individual AFP-positive (B) and negative (C) cells. Merged views are shown. (D-E) 2.5XD signal intensity profiles generated for AFP-positive (D) and negative (E) cells shown in (B-C). Merged views alongside individual channels for 5caC and DAPI are shown.