Figures & data
Figure 1. Flow chart of article screening and selection based on the template from PRISMA [Citation23]. ‘Second search’ refers to eligible studies published during the manuscript revision process.
![Figure 1. Flow chart of article screening and selection based on the template from PRISMA [Citation23]. ‘Second search’ refers to eligible studies published during the manuscript revision process.](/cms/asset/0d901c8e-7430-4121-be9a-69d033d02454/kepi_a_1903376_f0001_oc.jpg)
Table 1. Overview of the studies included in the literature review
Table 2. Overview of the methodology and statistical analysis
Table 3. Overview of genes examined in more than one candidate gene study on antidepressants
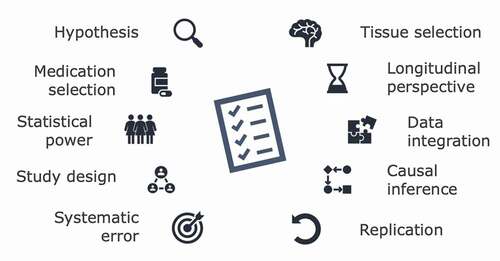
Box 1. (1)HYPOTHESIS: candidate gene studies should use a plausible hypothesis to guide the study design Hypotheses should be defined prior to designing a candidate gene study, and be guided by principles of teratology, knowledge of pharmacological mechanisms, and epidemiological and biological observations. Hypothesis-free EWASs are also important as the field of prenatal pharmacoepigenetic studies is still emerging.(2)MEDICATION SELECTION: investigate individual medications rather than medication classes Unless the pharmacological and epigenetic mechanisms of action of medications are expected to be similar across the medication class, medications should be analysed on an individual substance level.(3)STATISTICAL POWER: ensure sufficient sample sizes to detect relevant DNAm differences To detect biologically relevant DNAm associations and to ensure valid interpretation of the results, tools developed for power assessments in epigenetic studies should be used when planning such studies.(4)STUDY DESIGN: include a disease comparison group to disentangle medication from indicationStudies should include a disease comparison group to better differentiate the effects of exposure to medication from the underlying maternal disease. This may reduce the impact of confounding by indication.(5)SYSTEMATIC ERROR: assess selection bias, information bias, and confounding Selection bias should be assessed by comparing characteristics of study samples to the target population. The validity of medication exposure, neonatal phenotype, and other covariates should be reported, and information bias and misclassification addressed. Measured confounders of the exposure–outcome association(s) are to be adjusted for and residual confounding investigated. Importantly, cell type heterogeneity should be considered a confounding factor in epigenetic studies.(6)TISSUE SELECTION: biomarkers and extrapolation of DNAm patterns across tissues If the research aim is not only to report a tissue-independent biomarker, but to extrapolate results to other target tissues, the limitations of such translation should be recognized,, and reduced using software applications or data sets on cross-tissue correlations of modifications.(7)LONGITUDINAL PERSPECTIVE: assess persistence of DNAm patterns throughout childhoodThe follow-up of epigenetic patterns later in childhood is essential to assess the relevance of these changes over time, as they may suggest a long-term impact on the phenotypic outcome. (8)DATA INTEGRATION: integrate epigenetic data with complementary omics dataIntegration of complementary omics data, such as genomic and transcriptomic data, can strengthen functional and causal inferences of the findings. (9) CAUSAL INFERENCE: provides a framework for interpreting exposure-outcome associations Causal inference methods, such as two-step Mendelian randomization, may support the inference of causation from exposure–outcome associations, including how medication may impact phenotypic outcome via DNAm changes. Importantly, the underlying assumptions of causal methods are often untestable and, therefore, such methods should be used carefully.(10)REPLICATION: replicate findings using different methods and independent cohorts Replication both across methods and in independent cohorts is essential to increase the validity of the findings and the generalizability of the results to enhance clinical relevance..